Abstract
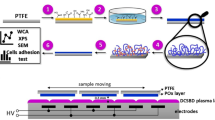
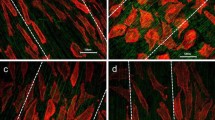
This study shows how surface properties of polydimethylsiloxane (PDMS) thin films, such as wettability and cellular adhesive behavior, can be influenced by surface modification and patterning, to improve the suitability of such modified PDMS for biomedical applications. For that purpose, different types of PDMS (i.e., soft (S-) and hard (H-) PDMS) and differently patterned surfaces were prepared by a commercially available tool which is originally used to produce patterned PDMS stamps for substrate conformal imprint lithography. To increase surface wettability and cellular adhesive behavior, PDMS surfaces were modified by O2, Ar/O2, Ar, and forming gas (N2/H2) plasma treatment. It is shown that, especially, plasma treatment using N2/H2 is a promising method to modify PDMS surfaces towards enhanced wettability. Such modified PDMS surfaces exhibit water contact angle values (WCA) of nearly 0° and demonstrate enhanced attachment of melanoma cells. Differences between as-prepared and plasma-treated S- and H-PDMS can be observed, where H-PDMS shows smaller WCA than S-PDMS. Even plasma-treated PDMS surfaces, however, exhibit only temporary hydrophilicity, due to hydrophobic recovery. To overcome this problem, plasma-treated PDMS films were stored in ethanol and water, where low and constant WCAs for several months could be achieved. Finally, the influence of surface topography of different patterned PDMS thin films on the adhesion behavior of melanoma cells was investigated. It can be concluded that chemical as well as structural modifications of PDMS enable to gradually control the adhesive properties of cells on their surfaces. This has a high technological impact since regulated and topologically stable adhesion and repulsion of cells by their scaffolds has high potency for in vitro as well as in vivo applications.

















Similar content being viewed by others
Notes
DataPhysics Interfacial Chemistry, Operating manual DataPhysics OCA, Data Physics Instruments GmbH, Germany
Diversified Enterprises (2008–2009) http://www.accudynetest.com/surface_tension_table.html?sortby=sort_st_disp. Accessed 28 July 2014
References
Yim, E., Reano, R., Pang, S., Yee, A., Chen, C., Leong, K. (2005). Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials, 26, 5405–5413.
Choi, H. G., Choi, D. S., Kim, E. W., Jung, G. Y., Choi, J. W., Oh, B. K. (2009). Fabrication of nanopattern by nanoimprint lithography for the application to protein chip. BioChip Journal, 3, 76–811.
Yao, J., Le, A. P., Gray, S. K., Moore, J. S., Rogers, J. A., Nuzzo, R. G. (2010). Functional nanostructured plasmonic materials. Advanced Materials, 22, 1102–1110.
Curtis, A. G. S., & Varde, M. (1964). Control of cell behavior: topological factors. Journal of the National Cancer Institute, 33, 15–26.
Flemming, R., Murphy, C., Abrams, G., Goodman, S., Nealey, P. (1999). Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials, 20, 573–588.
Truskett, V. N., & Watts, M. P. (2006). Trends in imprint lithography for biological applications. Trends in Biotechnology, 24, 312–317.
Tan, H. M., Fukuda, H., Akagi, T., Ichiki, T. (2007). Surface modification of poly(dimethylsiloxane) for controlling biological cells’ adhesion using a scanning radical microjet. Thin Solid Films, 515, 5172–5178.
Li, X., Wu, N., Rojanasakul, Y., Liu, Y. (2013). Selective stamp bonding of PDMS microfluidic devices to polymer substrates for biological applications. Sensors and Actuators A, 193, 186–192.
El Rassi, Z., Zhou, J., Ellis, A. V., Voelcker, N. H. (2010). Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis, 31, 2–16.
Rosenman, G., & Aronov, D. (2006). Wettability engineering and bioactivation of hydroxyapatite nanoceramics. 2006 NSTI Nanotechnology Conference and Trade Show - NSTI Nanotech 2006 Technical Proceedings, 2, 91–94.
Israelachvili, J. N. (2011). Intermolecular & surface forces (3rd ed.). San Diego: Elsevier Academic Press.
Tantra, R., & Cooper, J. (2002). Imaging of protein arrays and gradients using softlithography and biochip technology. Sensors and Actuators B, 82, 233–240.
Guo, L., Guvanasen, G. S., Liu, X., Tuthill, C., Nichols, T. R., DeWeerth, S. P. (2013). A PDMS-based integrated stretchable microelectrode array (isMEA) for neural and muscular surface interfacing. IEEE Transactions on Biomedical Circuits and Systems, 7, 1–10.
Tu, Q., Wang, J. C., Zhang, Y., Liu, R., Liu, W., Ren, L., et al. (2012). Surface modification of poly(dimethylsiloxane) and its applications in microfluidics-based biological analysis. Reviews in Analytical Chemistry, 31, 177–192.
Zhou, J., Khodakov, D. A., Ellis, A. V., Voelcker, N. H. (2012). Surface modification for PDMS-based microfluidic devices. Electrophoresis, 33, 89–104.
Bodas, D., Rauch, J. Y., Khan-Malek, C. (2008). Surface modification and aging studies of addition-curing silicone rubbers by oxygen plasma. European Polymer Journal, 44, 2130–2139.
Gomathi, N., Sureshkumar, A., Neogi, S. (2008). RF plasma-treated polymers for biomedical applications. Current Science, 94, 1478.
Fritz, J. L., & Owen, M. J. (1995). Hydrophobic recovery of plasma-treated polydimethylsiloxane. Journal of Adhesion, 54, 33–45.
Owen, M. J., & Smith, P. J. (1994). Plasma treatment of polydimethylsiloxane. Journal of Adhesion Science and Technology, 8, 1063–1075.
Hino, T., Igarashi, Y., Ymauchi, Y., Nishikawa, M. (2008). Surface wettability of silicon rubber after irradiation with a glow discharge plasma. Vacuum, 83, 506–509.
Verschuuren, M. A. (2010). Substrate conformal imprint lithography for nanophotonics. Dissertation, Utrecht University.
Fader, R., Schmitt, H., Rommel, M., Bauer, A. J., Frey, L., Ji, R., et al. (2012). Novel organic polymer for UV-enhanced substrate conformal imprint lithography. Microelectronic Engineering, 98, 238–241.
Schmid, H., & Michel, B. (2000). Siloxane polymers for high-resolution, high-accuracy soft lithography. Macromolecules, 33, 3042–3049.
Esteves, A., Brokken-Zijp, J., Laven, J., Huinink, H., Reuvers, N., Van, M., et al. (2009). Influence of cross-linker concentration on the cross-linking of PDMS and the network structures formed. Polymer, 50, 3955–3966.
Choi, K. M., & Rogers, J. A. (2003). A photocurable poly(dimethylsiloxane) chemistry designed for soft lithographic molding and printing in the nanometer regime. Journal of the American Chemical Society, 125, 4060–4061.
Mittal, K. L. (2009). Contact angle, wettability and adhesion (Vol. 6). Leiden: VSP/Brill.
Kaelble, D. H. (1970). Dispersion-polar surface tension properties of organic solids. Journal of Adhesion, 2, 66–81.
Owens, D. K. (1969). Estimation of the surface free energy of polymers. Journal of Applied Polymer Science, 13, 1741–1747.
Pinto, S., Alves, P., Matos, C. M., Santos, A. C., Rodrigues, L. R., Teixeira, J. A., et al. (2010). Poly(dimethyl siloxane) surface modification by low pressure plasma to improve its characteristics towards biomedical applications. Colloids and Surfaces, B: Biointerfaces, 81, 20–26.
Quéré, D. (2008). Wetting and roughness. Annual Review of Materials Research, 38, 71–99.
Sia, S. K., & Whitesides, G. M. (2003). Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis, 24, 3563–3576.
Wenzel, R. N. (1936). Resistance of solid surfaces to wetting by water. Industrial and Engineering Chemistry, 28, 988.
Cassie, A. B. D., & Baxter, S. (1944). Wettability of porous surfaces. Transactions of the Faraday Society, 40, 546–551.
Bushan, B., & Jung, Y. C. (2006). Micro- and nanoscale characterization of hydrophobic and hydrophilic leaf surfaces. Nanotechnology, 17, 2758–2772.
Lundgren, M., Allan, N. L., Cosgrove, T. (2003). A molecular dynamics study of wetting of a pillar-surface. Langmuir, 19, 7127–7129.
Lundgren, M., Allan, N. L., Cosgrove, T. (2007). Modeling of wetting: a study of nanowetting at rough and heterogeneous surfaces. Langmuir, 23, 1187–1194.
Extrand, C. W. (2004). Criteria for ultralyophobic surfaces. Langmuir, 20, 5013–5018.
Acknowledgments
We would like to thank Aaron Häring for preparing and analyzing of PDMS surfaces, Christian Matthus and Florian Stumpf for AFM analysis, and Philipp Singer for the SEM images. We are also grateful to Christian Maueröder for preparing and culturing of C57BL/6 mouse-derived B16F10 melanoma cells on PDMS. Additionally, we would like to point out the importance of the collaboration with Fraunhofer Institute for Integrated Systems and Device Technology (IISB), Germany, and the Department for Internal Medicine 3 and Institute of Clinical Immunology, University of Erlangen-Nuremberg, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scharin, M., Rommel, M., Dirnecker, T. et al. Bioactivation of Plane and Patterned PDMS Thin Films by Wettability Engineering. BioNanoSci. 4, 251–262 (2014). https://doi.org/10.1007/s12668-014-0145-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-014-0145-6