Abstract
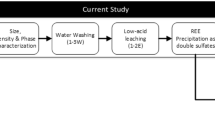
The RE-bearing slag used in the experiment was obtained from Ni–metal hydride battery wastes by H2 selective reduction and melting separation method, and the slag composition mainly consisted of 46.44 wt% REO, 26.05 wt% SiO2, 17.68 wt% Al2O3 and 6.32 wt% MnO. The influence of different reacting factors on the leaching rate of rare earth was investigated, and subsequently, the leaching dynamics was analyzed at low temperature and ordinary pressure and at high pressure and high temperature separately. The results showed that the optimal leaching parameters for the low-temperature and ordinary pressure conditions were 2.69 mol L−1 hydrochloric acid, 10:1 liquid to solid ratio at 85 °C for 75 min and the rare-earth leaching rate reaching 94.94%. According to Arrhenius formula, the apparent activation energy was 57.68 kJ mol−1 and the preexponential factor was 5.02 × 104 s−1, and the related reaction orders of the hydrochloric acid concentration and particle size were 3.63 and − 1.59, respectively. For the high-pressure and high-temperature leaching process, the optimal conditions were 2.49 mol L−1 hydrochloric acid, 10:1 liquid to solid ratio at 130 °C for 25 min and the rare-earth leaching rate 96.70%. The apparent activation energy analyzed was 53.63 kJ mol−1, and the preexponential factor was 5.32 × 103 s−1. The two leaching processes were both controlled by the interfacial mass transfer and solid film diffusion.










Similar content being viewed by others
References
Ahn N K, SwainB, Shim H W, and Kim D W, Metals 9 (2019) 1151.
Takeda O, Okabe T H, and Umetsu Y, J Alloys Comp 408–412 (2006) 387.
Abhilash A, Meshram P, Sarkar S, Venugopalan T, Min Metall Process 34 (2017) 178.
Huang W L, and Wang Z F, Metall Equip 4 (2016) 11.
Wang Y J, Liu Y H, Weng G Q, Li S Y, Liu R L, and Wang S L, Rare Metals Cem Carbides 35 (2007) 25.
Deng G F, Wu J P, Deng L L, Pan X B, Lin S H, and Huang L Q, Nonferrous Metals Sci Eng 8 (2017) 119.
Chen L J, Li Z L, Gong A, Tian L, and Xu Z F, J Chin Soc Rare Earths 37 (2019) 259.
Wang R, Yan J, Zhou Z, Zhou Z X, Deng B, and Gao X P, J Chin Soc Rare Earths 20 (2002) 138.
Saito T, Sato H, and Motegi T, J Mater Res 18 (2003) 2814.
Saito T, Sato H, Ozawa S, Yu J, and Motegi T, J Alloys Comp 353 (2003) 189.
Müller T, and Friedrich B, J Power Sour 158 (2006) 1498.
Tang K, Ciftja A, Van der Eijk C, Wilson S, and Tranell G, J Min Metall Sec B Metall 49 (2013) 233.
Elwert T, Goldmann D, Schirmer T, and Strauß K, ChemieIngenieurTechnik 86 (2014) 840.
Jiang Y J, Ma X K, Yang J C, Luo G P, Liu X D, and Song S K, Rare Earth 33 (2012) 47.
Jiang Y J, Song S K, Xu Z Y, Ma X K, Yang J C, Liu X D, and Luo G P, Rare Earth 33 (2012) 86.
Jiang Y J, Xu Z Y, Li P Z, Yang J C, Liu X D,Luo G P, Ma X K, Zhang F, Song S K, A Recycling Method of Waste Residue from AB5-type Alloy Production, ZL201010114005.9.
Deng Y C, Wu S L, Jiang Y J, Li H X, Rare Earth 36 (2015) 49.
Jiang Y J, Deng Y C, Bu W G, Metall Mater Trans B 46 (2015) 2153.
Deng Y C, Wu S L, Jiang Y J, Jia S Q, Metall Mater Trans B 47 (2016) 2433.
Deng Y C, Wu S L, Jiang Y J, Cui M M, Liu C, Chin J Process Eng 17 (2017) 86.
Deng Y C, Jiang Y J, Wang Y Q, Chin J Process Eng 17 (2017) 1203.
Jiang Y J, Deng Y C, Xin W B, Guo C, Trans Indian Inst Metals 73 (2020) 703.
Hua Y X, Introduction of metallurgical process kinetics, Metallurgy Industry Press, 2004.
Mo D C, Metallurgical kinetics, Central south university of technology Press, 1987.
Ma R J, Hydrometallurgical Principles, Metallurgy Industry Press, 2007.
Dickinson C F, Heal G R, Thermochimica Acta 340-341 (1999) 89.
Liu Z X, Yin Z L, Hu H P, Chen Q Y, J Central South Univ 19 (2012) 77.
Acknowledgements
The authors acknowledge financial support from the National Nature Science Foundation of China (Grant No. 51364029), the Natural Science Foundation of Inner Mongolia (Grant No. 2018LH05018) and by the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (Grant No. NJYT-20-B27).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Yc., Xin, Wb., Jiang, Yj. et al. Experimental Study on Recovering Rare Earths from Separation Slag of Ni–Metal Hydride Battery Wastes by Hydrochloric Acid Leaching. Trans Indian Inst Met 73, 2529–2538 (2020). https://doi.org/10.1007/s12666-020-02057-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02057-w