Abstract
This study aims to present a comparative assessment of hydrochemical characterization and groundwater quality in karst aquifers with the support of GIS which is essential to correlate the source of water with climate and geology, and to evaluate suitability of water for various uses. The study area is the Altinova region in Turkey where intensive agricultural activities prevail and travertine covers 85% of the study area. A total of 25 groundwater wells were monitored by seasonal field measurements and extensive water quality analyses for a period of one year to establish correlation between groundwater quality, its source, regional climate, and geology. A comprehensive analysis was conducted to assess the groundwater's suitability for irrigation based on electrical conductivity, sodium adsorption ratio, sodium percentage, magnesium hazard, permeability index, residual sodium bicarbonate, Kelly’s ratio, and an irrigation water quality (IWQ) index. This multi-parameter evaluation was further integrated with geospatial analysis using ArcGIS, providing a detailed spatial understanding of hydrochemical variations across the area. Major cations and anions dominance were identified as Ca2+ > Na+ > Mg2+ > K+ and HCO3− > Cl− > SO42−, respectively. Spatial mapping identified high concentrations of Ca2+, TDS, TH, and SO42− in non-karstic areas, occasionally exceeding WHO guidelines. Nitrate concentrations displayed varied spatial distribution. The SAR values generally matched C2-S1 and C3-S1 classes, suggesting medium to high salinity risks and low sodium presence. Based on the IWQ index and observed correlations with total dissolved solids, the groundwater in Altinova’s karstic aquifer is considered suitable for irrigation, with salinization largely due to ionic interactions and geology. The presented comparative assessment provides a holistic approach for understanding hydrochemical characteristics of karst aquifers, and analyzing the impacts of natural factors and anthropogenic pollution sources on groundwater quality.
Similar content being viewed by others
Introduction
Groundwater resources account for about one-third of the freshwater on the earth, and they play a key role in assuring food security for the increasing world population. Assessment of the groundwater hydrochemical characteristics is vital to ensure minimum health hazards as well as to maintain the quality for various uses. Irrigation water quality has been intensively investigated worldwide by many researchers such as Kumar et al. (2007) and Kumar and Al (2009) for the Patiala and Muktsar districts of Punjab, India, Delgado et al. (2010) for the karstic area of Yucatán in Mexico, Abbasnia et al. (2018) for some villages in Iran, Daanoba et al. (2019) for West Ghana and Zhang et al. (2019) for the Syr Darya River in Kazakhstan. In recent years, the focus around the globe was toward studying the effects of poor water quality on soil and crops’ yield. It is confirmed that highly saline–sodic water can affect the quality of soil by building soil salinity which in turn had adverse impacts on crop yields in many countries such as China and India (Pang et al. 2010; Adomako et al. 2011). Therefore, it is essential to ensure systematic monitoring of groundwater to prevent the deterioration of groundwater quality for beneficial uses. Groundwater quality was commonly evaluated using different irrigation water quality indices such as sodium adsorption ratio (SAR), magnesium adsorption ratio (MAR), total hardness (TH), sodium percentage (Na%), permeability index (PI), Kelly’s ratio (KR), Wilcox and US Salinity Laboratory (USSL) classifications (Zouahri et al. 2015; Province et al. 2015; Houatmia et al. 2016) which focused on single or very few number of parameters. However, the irrigation quality and the impacts on soil characteristics and crop yield is often a complex phenomenon which requires evaluation of the combined effect of many parameters (Ayers and Westcot 1985). Consequently, an irrigation water quality (IWQ) index was proposed by Simsek and Gunduz (2007) which takes into account five hazard groups of salinity, infiltration and permeability, specific ion toxicity, trace element toxicity, and miscellaneous impacts on sensitive groups. This IWQ index was applied to assess the irrigation water quality of the Simav Plain located in western Anatolia, Turkey and proved to be a fairly simple and practical tool. Another water quality index was developed by Meireles et al. (2010) using multivariate factor and principal component analysis which reflected soil salinity and sodicity risks to plants. Recently, entrophy weighted irrigation water quality index (EIWQI) was proposed and applied on Bharalu River to assess water quality for irrigation using derived parameters such as PI, KR, MAR, SAR, soluble Na%, and residual sodium carbonate (Singh et al. 2019).
Evaluation of the hydrochemical characteristics of the groundwater is crucial for water management at the regional level (Ben and Kamel 2009; Rajmohan and Senthamilkumar 2010; Aghazadeh and Mogaddam 2011; Sahraei and Nozar, 2013; Wang 2013; Bian et al. 2018; Gidey 2018; Omonona et al. 2019). Investigation of the hydrochemistry of the major, minor, and trace elements, geospatial distribution mapping, and statistical analyses are powerful techniques to assess aquifer pollution and address various natural and anthropogenic pollution sources affecting groundwater quality (El Alfy et al. 2018; Owoyemi et al. 2019; Saha and Paul 2019). Karst systems are particularly vulnerable to pollution from residential, agricultural, and industrial activities. Furthermore, groundwater resources originating from karst aquifers are under extreme deployment and over abstraction, especially in the Mediterranean region, due to adverse impacts of climate change. Therefore, effective land-use planning, proper groundwater management and conservation/adaptation plans are essential which should be based on a thorough hydrogeological characterization (Simsek et al. 2008). Hydrogeological and hydrogeochemical aspects of karst aquifers were commonly assessed using limited number of indices such as the use of USSL and Wilcox diagrams (Jebreen et al. 2018), mineral saturation indices, ion ratios, and oxygen isotope values (Liu et al. 2013) and irrigation suitability through SAR values (Nguyet et al. 2016). Consequently, this study aims to present a comparative assessment of hydrochemical characterization and groundwater quality in karst aquifers with the support of GIS which is essential to correlate the source of water with climate and geology, and to evaluate suitability of water for various uses. Karstic aquifers along the Mediterranean region of Turkey host significant amount of water that is primarily used for irrigation and domestic purposes. In Turkey, around 70% of the water use is allocated to agriculture, therefore, assessment of groundwater quality for both irrigation and domestic uses is crucial for the management of groundwater resources. Antalya is one of the main agricultural cities in the Mediterranean region of Turkey with a leading greenhouse applications that covers 53% of the total area of greenhouses in the country (Yilmaz et al. 2005). Despite the existing intensive agricultural practices, there is still little known about the irrigation water quality in the area. Furthermore, travertine karst regions of Antalya are facing variety of environmental pressures including the lack of proper wastewater collection system as well as the intensive and uncontrolled use of fertilizers and pesticides (Muhammetoglu et al. 2019). In some rural parts of Antalya province, municipal wastewater is directly discharged into the travertine aquifer via septic tanks causing contamination of groundwater resources. Likewise, this contamination may pose great risks that may extend to the food chain through the direct contact with the irrigated water. The existing studies mainly focused on the assessment of groundwater quality for domestic purposes in Antalya region. However, little is known about the agricultural water quality and its suitability for irrigation purposes. Therefore, this study was carried out over the karstic area of Altinova region of Antalya, located along the Mediterranean coast of Turkey, to assess the suitability of groundwater quality for irrigation by using several well-known irrigation water quality indices including the IWQ index. The novelty of the research study lies in its comprehensive evaluation of hydrochemical characteristics and groundwater quality in a karstic aquifer, based on several single and multi-parameter irrigation water quality indices such as SAR, Na%, residual sodium bicarbonate (RSB), PI, MAR, KR, and IWQ index. By conducting a comparative assessment of hydrochemical characterization and groundwater quality, the research contributes valuable insights into the suitability of groundwater for irrigation purposes in intense agricultural lands.
Materials and methods
Study area
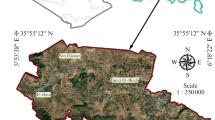
Antalya is a metropolitan city located along the Mediterranean coast in the southwest of Turkey (Fig. 1) and covers an area of 20,723 km2. The area of Altinova is surrounded by the Taurus Mountains at the southeastern Anatolia region and runs from the east to the western part of the Mediterranean Sea. The pilot study area (PSA) of Altinova is located approximately 10 km northwest of Antalya City center. The PSA covers an area of approximately 74 km2 and is situated on a plain where the mountains recede southward forming flat areas of a travertine plateau. The area is in the northwest border of Antalya international airport between the coordinates of 36°54′ and 37°2′ northern latitudes, 30°43′ and 30°50′ eastern longitudes. The topography of the region ranges between less than 50 m to more than 300 m above the mean sea level from the southern to the northern border, respectively (Fig. 1).
The study area is a home for farming activities where intensive agriculture in the form of greenhouses is practiced almost across the entire area and the total area of the greenhouses is estimated at 17.4 km2. Groundwater which recharges only during the rainy season (November–February) of the year is the main source of irrigation and most of the farmers have private water wells for irrigation. Industrial and residential areas in the PSA also depend on the groundwater for their daily use. The main pervasive pollutants in the PSA are from anthropogenic sources such as municipal wastewater discharged into the aquifer through septic tanks, fertilizers and pesticides used in agriculture in addition to domestic and industrial wastes. The PSA has a Mediterranean climate with mild, rainy winters, hot and dry summers. According to the meteorological data collected from the nearest meteorological station (Antalya Airport) for the years between 1994 and 2017, the average yearly precipitation of the PSA is about 1191.5 mm/year, while the mean and maximum air temperatures ranged between 25 and 35 °C in summer and 14 to 17 °C in winter, respectively.
Geological and hydrogeological settings
The autochthonous and tertiary formation of the PSA is mainly composed of travertine, conglomerates, and alluvium of the Kursunlu formation. Approximately 63 km2 of the study area is covered by the karst travertine formation which accounts for about 85% of the PSA. The Kursunlu formation consists of conglomerates and sandstone which mostly covers the eastern part of the study area and accounts for approximately 14%. The third geological formation is the alluvium and conglomerates which cover only 1% as per the geological map of the study area (Fig. 2a). The thickness of the travertine varies between around 240 m in the upper plateau down to around 100 m in the lower plateau, while it is around 50 m in the northeastern part of Antalya (Efe et al. 2008). The thickness of both the Kursunlu and the travertine formations in the PSA ranges between 30 and 180 m.
a Geological formation of the PSA, b groundwater flow direction and c extended cross section of the PSA (after Davraz 1998 as cited in Davraz et al. 2008)
A total of 25 representative irrigation groundwater wells were chosen for monitoring of groundwater quality and depth to groundwater table at the PSA, as depicted in Fig. 1. According to hydrological measurements conducted in November 2015, February and May 2016, the average depth to groundwater table varied between 14.3 m and 43.5 m (Muhammetoglu et al. 2017; Wani 2019). Based on the geotechnical investigations, yield of water wells varied between 1 and 100 m3/s, as reported during the time of drilling (DSI 2005). Net recharge is estimated by around 44% of the precipitation in the karstic zone of the study area, whereas approximately 15% of the precipitation infiltrates to the aquifer in the non-karstic zone of the PSA (AMPR 2016). The groundwater flows from the north to the south in the PSA as depicted in Fig. 2b (Wani 2019). The extended cross section of the PSA is presented in Fig. 2c.
Monitoring of groundwater quality
The locations of the 25 monitoring wells were documented by means of GPS. The physicochemical characteristics of the groundwater samples were determined by field measurements and laboratory analyses following the standard procedures. Four sessions of seasonal field measurements were conducted at the monitoring wells for temperature, salinity, specific conductivity (SC), pH, total dissolved solids (TDS), turbidity, and dissolved oxygen (DO) in August and November 2015, February and May 2016 using the HACH HQ40d digital multi-parameter instrument and HACH 2100Q-is turbidity meter. Nitrate analyses were conducted in the laboratory of the Environmental Engineering Department at Akdeniz University using the HACH DR5000 spectrophotometer and HACH Lange LCK 339 nitrate cuvette test (2.6-Dimethylphenol, Standard method: EN 38405 D-2 method). Two sessions of intensive water quality sampling were realized in November 2015 and May 2016 at all the monitoring wells for many physical, bacteriological and chemical parameters. The analyses of all parameters were conducted at the accredited Turkish Ministry of Health, Antalya Public Health Laboratory. Prior to sampling of groundwater, the well water was pumped multiple times to collect freshwater samples. The samples were obtained from private wells with sampling depths ranging from 14 to 44 m. Polyethylene bottles were initially cleaned by the groundwater itself through a threefold cleaning process before being used for collection. A volume of 2 L of groundwater was collected for the analysis of ions and trace elements. To prevent interference from atmospheric gases, all bottles were filled with groundwater and securely sealed with parafilm. Pre-sterilized 100 mL amber glass sample bottles were utilized for collecting samples for bacteriological parameters. The water samples were stored in a cooler box at a low temperature (approximately 4 °C) and kept in the dark until transported to the laboratory. Analysis of major anions/cations and trace elements was carried out using Ion Chromatography and Inductively Coupled Plasma Mass Spectrometry (ICP-MS). Bicarbonate analysis employed titrimetric methods. Total coliform and Escherichia coli (E. coli) analyses were performed using the membrane filtration technique according to the TS EN ISO 9308-1 method. Each water sample underwent triplicate analysis, with standards and blanks regularly dispersed in sample batches to ensure data quality. To ascertain the reliability of the analyses results, the ionic charge balance percentage criterion was performed for each well where the ionic balance error was generally within ± 5%.
Methodology of the study
The methodology employed in this study, as briefly outlined in Fig. 3, consists of three distinct phases. In the first phase, a monitoring study was conducted, involving both field measurements and comprehensive analyses of water quality parameters. Subsequently, in the second phase, the evaluation of monitoring study outcomes was undertaken through a comparative assessment. This involved the evaluation of irrigation water quality and the examination of hydrochemical facies, along with the classification of water quality based on USSL, Wilcox, and Doneen’s diagrams. Correlation analysis of physicochemical parameters and the application of the irrigation water quality (IWQ) index were integral to this phase. The results obtained from the IWQ index were classified following the methodology proposed by Simsek and Gunduz (2007). In the final phase, spatial variations in groundwater quality parameters were thoroughly assessed utilizing a geospatial analysis tool within the GIS environment. The integration of GIS facilitated the coherent classification of regions with analogous water quality indices, thereby enhancing the visibility of spatial analyses and streamlining the decision-making processes for stakeholders.
Evaluation of irrigation water quality
The groundwater quality parameters used for assessing the suitability of groundwater for irrigation include Electrical Conductivity (EC), SAR, Na%, RSB, PI, MAR, KR and trace elements. Additionally, US Salinity Laboratory USSL diagram was plotted, based on computed SAR values and measured EC, to determine the suitability of the groundwater for irrigation. The groundwater suitability was computed for each monitoring well using the equations presented in Table 1. The concentrations of major anions and cations as percentages of milliequivalents per liter (meq/L) were plotted in the Piper diagram (Piper 1944). This was done to give better understanding of the hydrochemical processes in the aquifer system and to identify groundwater type in the PSA.
The relationship between different physicochemical variables was determined using correlation analysis where the correlation coefficient (r) higher than 0.5 was classified as significant. The spatial variation of groundwater quality was assessed by geospatial analysis tool in the GIS environment. The spatial database was integrated using ArcGIS, in order to generate spatial distribution maps of the suitability indices. The spatial interpolation method employed to delineate the distribution maps of groundwater parameters was ordinary kriging.
Irrigation water quality (IWQ) index
In this study, the IWQ index, developed by Simsek and Gunduz (2007) was applied where five groups of hazard including (1) salinity, (2) permeability, (3) specific ion toxicity, (4) trace element toxicity and (5) miscellaneous impacts on sensitive groups were included (Tables 2 and 3). The water quality parameters of these groups were selected based on the guidelines presented by Ayers and Westcot (1985). These five groups of hazard were assigned a weight value from highest (5) to lowest (1), based on their significance in irrigation water quality. It is well established in the literature that the salinity hazard is a significant water quality factor that influences the soil permeability therefore a weight of (5) was assigned to the salinity hazard. On the other hand the least significant factor including the miscellaneous effect was given the lowest weight (1). To compute the IWQ index, the contribution of each parameter was considered and a suitable rating value was assigned. The general formula used in the calculation of IWQ index is given in Eq. 1 (Simsek and Gunduz 2007):
where i is an incremental index and G represents the contribution of each of the five hazard groups. The influence of each group of hazard G was calculated using Eq. 2.
where k is an incremental index, N is the total number of parameters for the calculation, w is the weight value of the selected groups, and r is the rating value of each parameter (Tables 2 and 3). Based on the computed total value of the index, the suitability evaluation is done according to the criteria presented by Simsek and Gunduz (2007) where the values of IWQ index < 22, 22 < IWQ index < 37 and IWQ index > 37 indicate low, medium and high suitability of water for irrigation, respectively. The details for generating irrigational suitability index values and grid processing procedures in GIS are explained elsewhere (Simsek and Gunduz 2007).
Results and discussion
Hydrochemical characteristics of the groundwater
The descriptive statistics of the monitored parameters are presented in Table 4 along with the guideline levels of the World Health Organization (WHO) for drinking water quality (WHO 2006). The groundwater temperature (T) was in the range of 17.7–24 °C with an average of 20.13 °C. According to Daanoba et al. (2019), such temperature values propose that there is no hydrothermal influence in the aquifer. The measured values of pH in the PSA varied between 6.44 and 7.54 with an average value of 7.10 which is classified as neutral to weak alkalinity. The EC and TDS values had wide ranges with average values of 665.96 µS/cm and 429.33 mg/L, respectively. The recommended guideline range for electrical conductivity (EC) was set between 500 and 700 µS/cm, where 40% of the groundwater samples exhibited measured EC values ranging from 418 to 948 µS/cm. Some of the monitoring wells exhibited elevated concentrations of TDS exceeding the WHO guideline value of 500 mg/L. Dominance of major cations in the groundwater samples was in the order of Ca2+ > Na+ > Mg2+ > K+ and all the values were within the limits of WHO guidelines. The concentration of Na+ and K+ in the groundwater samples varied from 4.85 to 24.14 mg/L and 0.31 to 3.62 mg/L with average values of 12.52 and 1.14 mg/L, respectively. The K+ concentration in the groundwater samples was relatively low in the entire PSA. TH in the PSA ranges between 195.13 and 440.71 mg/L as CaCO3 with an average value of 307.88 mg/L. Hardness is classified as soft (< 75 mg/L), moderate (between 75 and 150 mg/L), hard (between 150 and 300 mg/L) and very hard (> 300 mg/L as CaCO3). Accordingly, hardness level was classified as hard for 52% and very hard for 48% of groundwater samples in the PSA where Ca2+ was the predominant cation resulting from the soil lithology and the strong dissolution of carbonate rocks. Although the high TH levels may not pose potential risk to public health, it is generally not preferred by the local people for drinking. The level of the key anions in the PSA was in the order of HCO3− > Cl− > SO42− and HCO3− was the main anion with a mean value of 278 mg/L. These high values of HCO3− might be due to the autochthonous nature of the PSA which is predominantly limestone and sandstones. The F− concentrations were also relatively low ranging from 0.04 to 0.17 mg/L with a mean value of 0.08 mg/L. The monitoring results showed that approximately 96% of the groundwater samples had high TH and HCO3− values which exceeded the guideline values of WHO. The monitoring results revealed high NO3− concentrations with a mean value of 45.75 mg/L, and occasional high values, exceeding 100 mg/L, at two monitoring wells namely K18 and K20. This high level of nitrate concentrations was more than twice the recommended guideline value of NO3− by the WHO. Approximately 48% of the groundwater samples exhibited high NO3− concentrations above the WHO standard of 50 mg/L which was mainly due to the disposal of municipal wastewater by septic tanks and intense use of fertilizers, posing serious risk to the groundwater quality (Muhammetoglu et al. 2017). The results of water quality analyses for trace elements is given in Table 5 where low concentrations of As3+ were observed in general with an occasional maximum value of 26.42 µg/L.
Spatial distribution of hydrochemical parameters and coliform bacteria
The spatial distribution of the hydrochemical parameters and coliform bacteria are presented in Fig. 4 and Figure S1 (in the Supplementary File). The spatial maps revealed that the non-karstic aquifer has higher levels of Ca2+, Na+, TDS, TH, and SO4−2 than the karstic part, where some of the monitoring results exceeded the WHO guidelines for drinking water. NO3− concentrations showed high spatial variations in the range of less than 1 mg/L to more than 100 mg/L. High NO3− values were observed in 12 monitoring wells exceeding the acceptable limit of 50 mg/L. The highest nitrate levels were encountered mostly in the dense agricultural and residential areas which could be due to intensive usage of fertilizers and direct disposal of the municipal wastewater into the aquifer. High levels of EC (> 700 µS/cm), above the WHO guidelines, were observed in the monitoring wells of K1, K2, K3, K4, K19, K20, and K24 in the non-karstic formation. High levels of F− and K+ were observed in the southwestern part of the PSA within the karstic zone. Relatively high numbers of coliform bacteria were monitored in the entire PSA due to existence of many septic tanks where the maximum number of coliforms was analyzed as 3000 cfu/100 mL at the monitoring well of K11.
Mechanisms controlling water chemistry
The Gibbs diagram is a valuable tool for interpreting the geochemical processes influencing groundwater chemistry and understanding the hydrogeochemical evolution of groundwater systems. This diagram shows the relationship between TDS and Na + K/(Na + K + Ca) and represents the weight ratio of TDS to Cl/(Cl + HCO3). The visualization of all samples in the rock dominance field in the Gibbs diagram shows that the factor that controls groundwater chemistry is the rock–water interaction (Fig. 5). This phenomenon is indicative of potential rock weathering and mineral dissolution, both of which are likely contributing factors to the observed changes in water chemistry. The outcomes of these interactions emphasize the significance of understanding and considering the geological context when evaluating water quality and its associated chemical composition.
Hydrochemical facies
Investigation of hydrochemical facies enhance the identification of chemical processes occurring within the lithologic framework and the groundwater flow pattern through the aquifer systems (Piper 1944; Glynn and Plummer 2005). The Piper diagram and the Hydrochemical Facies Evolution-Dominance (HFE-D) (Gimenez-Forcada 2010) method are well-known tools used in hydrochemical facies analysis. In the study of Mirzavand and Ghazban (2022), HFE-D method was applied to provide information on salinization and freshening processes for groundwater salinization in Kashan Plain aquifer in Iran. The choice between the Piper diagram and HFE-D method depends on the specific goals of the hydrochemical facies analysis. The HFE-D method is valuable for temporal analysis and a quantitative understanding of hydrochemical facies evolution while the Piper diagram is advantageous for clear and concise visual representation of water chemistry and comprehensive classification of dominant ions. As the Piper diagram is a well-established and widely used method for hydrochemical analysis, it was selected to examine the hydrochemical facies of groundwater in the PSA and the concentrations of major anions and cations were plotted in the Piper diagram (Fig. 6). The plot revealed that major hydrochemical facies of groundwater were dominated by bicarbonate type, calcium type and magnesium bicarbonate type with an insignificant trace of chloride type whereas Na + K cation facies were not identified. There was no distinct difference in terms of hydrochemical facies for the monitoring wells in the karstic and non-karstic parts of the PSA due to similarities in chemical processes and composition. Generally, CaHCO3 and MgHCO3 facies were dominant within the PSA where most of the monitoring wells were in the field of alkaline Earth metal (Ca2+, Mg2+) dominating over the alkalies (Na+, K+). It is important to mention that similar water type was reported in an early study conducted at Antalya Basin, covering the PSA, by Davraz et al. (2008). Consequently, the dominant water type in the autochthonous karstic unit of Turkey (Antalya) is described as CaHCO3 type.
Correlation matrix
Correlation analysis is a useful tool to identify the relationship between physicochemical characteristics of groundwater and to elaborate the geochemical processes that contribute to the groundwater mineralization and composition, as presented in Table S1 (in the Supplementary File). In general, the leaching of limestone is the main source of Ca2+ and Mg2+ ions present in the groundwater. Calcium ions can be also released from the cation exchange reactions (Kumar et al. 2009). The relationship between the ground elevation and EC was investigated for the PSA (Figure S2 in the Supplementary File) where the results revealed that the EC in the water samples increased with the decrease of the elevation as the groundwater moved toward lower elevations in the south. These findings are in agreement with the study conducted in a similar environment at Nif Mountain by Simsek et al. (2008). In addition to the rock–water interactions, agricultural activities including irrigation and fertilizer usage in the PSA are likely the main factors affecting changes in groundwater quality and, consequently, impacting the EC level. According to the results of correlation analysis, pH had significant correlation with EC, TDS, salinity and the ions of Ca2+, Na+ and SO4−2. Additionally, high correlations were obtained between EC and many major ions including Ca2+ (r = 0.9), Mg2+ (r = 0.6), Na+ (r = 0.8), HCO3− (r = 0.6), and SO42− (r = 0.7) indicating a similar source for these parameters.
The correlation between Ca2+ and HCO3− (r = 0.5) suggested that calcite was the main sources of Ca2+ and HCO3− in the groundwater which was probably derived from carbonate weathering in the aquifer. Furthermore, a significant correlation between Ca2+ and SO42− was observed (r = 0.7) indicating that calcium and sulfate were associated with mineralization of the groundwater in the PSA. This further suggested that the main source of Ca2+ in the groundwater results from the dissolution of carbonate rocks (limestone) which is mostly related to Mesozoic units in the PSA. HCO3− exhibits a high correlation between both Na+ (r = 0.7) and Mg2+ (r = 0.6) implying the possible dissolution of carbonates rocks within the aquifer. The highest and positive correlations were observed between TDS and Salinity, EC and TDS, Salinity and EC (r = 1.0). The SAR values had a significant correlation with Na+ (r = 1.0), EC and TDS (r = 0.8).
Groundwater quality assessment for irrigation purpose
Numerous indices were used in the literature for evaluating the groundwater suitability for irrigation usage where the investigation was based on various ions and cations. In this research study, the salinity level in terms of EC (Richard 1954; Raghunath 1987) and many irrigation water quality indices including SAR, PI, MAR, KR, Na %, RSB, in addition to US Salinity Laboratory USSL diagram were used to assess the suitability of the groundwater in the PSA for irrigation purpose. The descriptive statistics of the irrigation water quality indices is shown in Table 6 for all the monitoring wells in the PSA whereas the evaluation of groundwater quality for irrigational use is presented in Table 7.
SAR is a widely used indicator for measuring both surface and groundwater suitability for irrigational use as it indicates the alkali/sodium hazard to crops. The excess Na+ leads to the decrease in Ca2+ and Mg2+ since the soil Na+ uptake can replace these ions. Food and Agriculture Organization (FAO) recommends the guideline value of SAR to be between 0 and 15 (Ayers and Westcot 1985). All the levels of SAR in the PSA were within the allowed range for irrigational use. The low SAR levels in the PSA might be associated with the alkaline soil lithology. To further interpret the results, the computed SAR and the measured EC values were plotted in the USSL diagram (Fig. 7a) (Richards 1954). The results showed that the computed SAR values ranged from 0.14 to 0.56 with an average value of 0.30 where all the results were within C2-S1 and C3-S1 classes. 68% of the groundwater samples were within C2-S1 class indicating a low sodium hazard with medium salinity level, whereas the remaining 32% of the samples, which were within C3-S1 class, indicated a relatively high salinity hazard with low sodium hazard.
Na% is extensively used for determining the suitability of groundwater for irrigation where high Na+ level in water can negatively impact the soil permeability and, hence, limit the flow of air and water. Figure 7b shows the spots of the distinct groundwater types in the Wilcox diagram (Wilcox 1955) where the groundwater samples were classified as excellent and good. According to Na% index, groundwater in the PSA is excellent for irrigation purpose. The Na% values varied from 4.59 to 13.47 with an average of 8.21. FAO recommends the guideline value of EC to be below 3000 µS/cm (Ayers and Westcot 1985) and the EC values in the PSA showed a wide range between 418 and 948 µS/cm, suggesting that the groundwater samples were within the acceptable range for irrigation water quality (Ayers and Westcot 1985). Approximately 68% of the groundwater samples had a good level of EC whereas the remaining 32% of samples, taken from the non-karstic area (monitoring wells of K1, K2, K9, K19, K20, K21, K23 and K24), had permissible values of EC between 750 and 950 µS/cm due to intense anthropogenic activities. From irrigation point of view, the results revealed that the current groundwater quality is safe and suitable for irrigation purpose in the karstic part of the PSA with limited to no sodium hazards. However, mixing of groundwater with other sources of freshwater is highly recommended to reduce the salinity level for the non-karstic part of the PSA for irrigational use or groundwater in that part could be used to irrigate relatively high salt-tolerant plants.
MAR is also an essential index in evaluating the groundwater suitability and MAR indicates a harmful impact on the soil when it exceeds 50 (Raghunath 1987). At the same level of salinity and SAR, adsorption of Na+ by soil and clay minerals increases when the ratio of Mg2+ to Ca2+ increases, due to weak bonding energy of Mg2+ compared with that of Ca2+. The higher uptake of Na+ occurs particularly if this ratio is greater than 4. The computed values of MAR in all the groundwater samples were in the range of 3.10–27.40 with an average of 7.80 (Table 6) which revealed that the groundwater is suitable for irrigation. KR is among the most important parameters for determining the hazardous effect of sodium on groundwater quality which indicates the balance among Na+, Ca2+, and Mg2+ ions in water. Water samples with KR values greater than 1 is considered unsuitable for irrigation. The values of KR in the groundwater samples were in the range of 0.046–0.150 with an average value of 0.086 (Table 6) which revealed that the groundwater is suitable for irrigation. In case of PI, the groundwater samples were in the range of 29.20–48.36 and they were classified as suitable for irrigation. Based on the relationship between PI values and total concentration of Na+ and HCO3−, as plotted in the Doneen’s diagram (1964), the groundwater quality was found as Class I, being suitable for irrigational use in almost all types of soil (Fig. 7c). The groundwater in the PSA showed a high level of quality and suitability for irrigation purpose based on the PI.
The spatial distribution maps of different irrigation water quality indices, as presented in Fig. 8, revealed that the groundwater in the PSA is suitable for irrigation purpose in regard to all irrigation indices such as SAR, Na%, MAR, PI, KR, and RSB; whereas both good and permissible levels were obtained according to EC. The spatial distribution of SAR in the PSA was within the acceptable range, where the high values appeared in the west and southeast parts of the PSA. The spatial distribution map of Na% showed relatively high levels of Na+ for the monitoring wells located at the southeastern and western parts of the PSA which is mainly due to the land-use activities.
Results of IWQ index
It is well known that comparative assessment based on single or limited number of parameters cannot give in-depth characterization of the water quality for different uses. The current study investigated most of the well-known irrigation water quality indices for the PSA. However, IWQ index, which takes into account many parameters in addition to trace elements, was also applied to the PSA and the spatial distribution map of IWQ index was created based on the computed index values (Fig. 9). The computed IWQ index values were in the range of 38.98–40.64, where the suitability is classified as high. The spatial analysis of IWQ index values revealed that all the PSA had high suitability class of irrigation with minor spatial differences. Consequently, based on the overall IWQ index values, the groundwater quality in the autochthonous formation of karstic and non-karstic aquifer of the PSA was evaluated to be suitable for irrigational use. However, special care should be given toward protecting the groundwater resources from any potential deterioration particularly due to the current practices of the intensive use of agricultural fertilizers and the disposal of wastewater into the aquifer.
The parameters and irrigation water quality criteria classification of the IWQ index complies well with the criteria outlined in the Draft Regulation on “Quality of Irrigation Water and Reuse of Used Water” by the Turkish Ministry of Agriculture and Forestry. The General Irrigation Water Quality criteria given in the regulation comprises the same parameters presented in Tables 2 and 3 with three classes of use restrictions (no restriction, low-medium level of restriction, high level of restriction). Therefore, IWQ index is a practical tool to evaluate multi parameters of groundwater quality in a single index value for irrigation water quality assessment.
Conclusion
Despite the significant role of karstic aquifers in supplying water for both irrigation and domestic purposes in the Mediterranean region, there is a lack of knowledge regarding the quality of water used for irrigation, especially in areas like Antalya with intense agricultural practices. This study addresses this gap by employing various well-established irrigation water quality indices, including SAR, Na%, RSB, PI, MAR, KR, and the IWQ index. The study was applied in the PSA of Altinova region with an autochthonous karst aquifer. The PSA is covered with Cenozoic formation, composed of karstic travertine, alluvium of quaternary and clay, sandstones, and conglomerate of tertiary formation. The dominance of the major cations and anions in the groundwater was as follow: Ca2+ > Na+ > Mg2+ > K+ and HCO3− > Cl− > SO42−. Generally, the dominant water type in the PSA was CaHCO3 facies where most of the groundwater samples were in the field of alkaline Earth metals (Ca2+, Mg2+). According to the USSL diagram, the groundwater samples were within C2-S1 and C3-S1 classes, highlighting medium to high salinity hazard and low sodium content. Additionally, other irrigation water quality indices, such as Na%, MAR, PI, RSB, and KR showed that groundwater quality at the PSA is suitable for irrigational use. The calculated indices were all within the recommended limits of US Salinity Laboratory (USSL). The concentration of the physicochemical elements (cations and anions) were within the normal range of irrigation water quality. However, more attention toward the elevated levels of EC is required mainly in the non-karstic part of the PSA as high salinity can negatively affect the soil and crops. Major cations and anions have a strong relationship with the TDS indicating that the salinization of groundwater is due to the increased ionic concentration resulting from the interactions between the groundwater and the geological formation. In case of IWQ index, the groundwater in the PSA was evaluated to have high suitability for irrigational use. Comparatively high levels of NO3−, TDS, EC, TH, HCO3−, and coliform bacteria were monitored in the intense agricultural areas and residential areas that lack proper wastewater collection and disposal systems. In conclusion, hydrochemical characterization and assessment of groundwater are essential processes that provide insights into the quality, quantity, and sustainability of groundwater resources. The use of stable nitrogen isotopes (15N:14N) is recommended for future research studies aimed at distinguishing between natural/anthropogenic sources of nitrate, understanding nitrogen transformation processes, exploring temporal variability and comprehending groundwater flow dynamics.
Data availability
The data that support the findings of this study are available from the corresponding author, Firdes Yenilmez, upon reasonable request.
References
Abbasnia A, Radfard M, Mahvi AH, Nabizadeh R, Yousefi M, Soleimani H, Alimohammadi M (2018) Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar Sistan and Baluchistan, Iran. Data Brief 19:623–631
Adomako D, Gibrilla A, Akiti TT, Fianko R, Maloszewski P (2011) Hydrogeochemical evolution and groundwater flow in the Densu River Basin, Ghana. J Water Resour Protect 3:548–561
Aghazadeh N, Mogaddam AA (2011) Investigation of hydrochemical characteristics of groundwater in the Harzandat aquifer, Northwest of Iran. Environ Monit Assess 176:183–195
AMPR (2016). Antalya Master Plan Report, Hydrogeology Report, State Hydraulics Works, Antalya Turkey
Ayers RS, Westcot DW (1985) Water quality for agriculture, FAO Irrigation and Drainage Paper No. 29, Rev. 1, United Nations, Food and Agriculture Organization, Rome
Ben A, Kamel M (2009) Geochemical study of groundwater mineralization in the Grombalia shallow aquifer, north-eastern Tunisia: implication of irrigation and industrial waste water accounting. Environ Geol 58(3):555–566
Bian J, Nie S, Wang R, Wan H, Liu C (2018) Hydrochemical characteristics and quality assessment of groundwater for irrigation use in central and eastern Songnen Plain, Northeast China. Environ Monit Assess 190:382. https://doi.org/10.1007/s10661-018-6774-4
Daanoba E, Abu M, Suuchullo P (2019) Hydrogeochemical appraisal of groundwater quality in the Ga west municipality, Ghana: implication for domestic and irrigation purposes. Groundw Sustain Dev 8:501–511
Davraz A, Karaguzel R, Soyaslan I, Sener E, Seyman F, Sener S (2008) Hydrogeology of karst aquifer systems in SW Turkey and an assessment of water quality and contamination problems. Environ Geol 58:973–988
Delgado C, Pacheco J, Cabrera A, Batllori E, Orellana R, Bautista F (2010) Quality of groundwater for irrigation in tropical karst environment: the case of Yucatán, Mexico. Agric Water Manag 97(10):1423–1433
Doneen LD (1964) Water quality for agriculture, Department of Irrigation. University of California, Davis, p 48
DSI (2005) Antalya Kırkgozler Project Preliminary Investigation Report, State Hydraulics Works, p 21 (in Turkish)
Efe R, Atalay I, Soykan A, Cürebal I, Sarı C (2008) The formation of Antalya travertine deposit and karstic ground water systems, Environment and Culture in the Mediterranean Region Part I, Chapter Six, 93–108. Cambridge Scholars Publishing, Newcastle
El Alfy M, Alharbi T, Mansour B (2018) Integrating geochemical investigations and geospatial assessment to understand the evolutionary process of hydrochemistry and groundwater quality in arid areas. Environ Monit Assess 190:277. https://doi.org/10.1007/s10661-018-6640-4
Gidey A (2018) Geospatial distribution modeling and determining suitability of groundwater quality for irrigation purpose using geospatial methods and water quality index (WQI) in Northern Ethiopia. Appl Water Sci 8:82. https://doi.org/10.1007/s13201-018-0722-x
Gimenez-Forcada E (2010) Dynamic of seawater interface using hydro-chemical facies evolution diagram (HFE-D). Groundwater 48(2):212–216. https://doi.org/10.1111/j.1745-6584.2009.00649.x
Glynn PD, Plummer LN (2005) Geochemistry and the understanding of groundwater systems. Hydrogeol J 13:263–287
Gupta IC (1983) Concept of residual sodium carbonate in irrigation waters in relation to sodic hazard in irrigated soils. Curr Agric 7:97–113
Houatmia F, Azouzi R, Charef A, Be M (2016) Assessment of groundwater quality for irrigation and drinking purposes and identification of hydrogeochemical mechanisms evolution in Northeastern, Tunisia. Environ Earth Sci 75:746. https://doi.org/10.1007/s12665-016-5441-8
Jebreen H, Wohnlich S, Banning A et al (2018) Recharge, geochemical processes and water quality in karst aquifers: Central West Bank Palestine. Environ Earth Sci 77:261. https://doi.org/10.1007/s12665-018-7440-4
Kelley WP (1963) Use of saline irrigation water. Soil Sci 95(6):385–391
Kumar M, Kumari K, Ramanathan A, Saxena R (2007) A comparative evaluation of groundwater suitability for irrigation and drinking purposes in two intensively cultivated districts of Punjab, India. Environ Geol 53:553–574
Kumar M, Kumari K, Singh UK, Ramanathan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57(4):873–884
Liu X, Li L, Hu A (2013) Hydrochemical characterization of a groundwater aquifer and its water quality in relation to irrigation in the Jinghuiqu Irrigation District of China. Water Environ Res 85(3):245–258
Meireles A, Andrade EM, Chaves L, Frischkorn H, Crisostomo LA (2010) A new proposal of the classification of irrigation water. Rev Ciênc Agron 41(3):349–357
Mirzavand M, Ghazban F (2022) Isotopic and hydrochemical evidence for the source and mechanism of groundwater salinization in Kashan Plain aquifer in Iran. Environ Sci Pollut Res 29:34575–34593. https://doi.org/10.1007/s11356-021-17457-8
Muhammetoglu A, Ozyurt NN, Muhammetoglu H, Yenilmez F (2017) Integrated management of groundwater quality in agricultural karstic regions using groundwater risk assessment and pesticide risk indicators, Project Final Report, Antalya, Turkey
Muhammetoglu A, Keyikoglu R, Cil A, Muhammetoglu H (2019) Integrated management of pesticides in an intensive agricultural area: a case study in Altinova, Turkey. Environ Monit Assess 191:599. https://doi.org/10.1007/s10661-019-7748-x
Nguyet VTM, Nguyet VTM (2016) Hydrogeochemical characteristics of karst groundwater in Tam Diep, Ninh Binh, Vietnam. Acta Carsol. https://doi.org/10.3986/ac.v45i3.3588
Omonona OV, Amah JO, Olorunju SB, Waziri SH, Ekwe AC, Umar DN, Olofinlade SW (2019) Hydrochemical characteristics and quality assessment of groundwater from fractured Albian carbonaceous shale aquifers around Enyigba-Ameri, southeastern Nigeria. Environ Monit Assess 191:125. https://doi.org/10.1007/s10661-019-7236-3
Owoyemi FB, Oteze GE, Omonona OV (2019) Spatial patterns, geochemical evolution and quality of groundwater in Delta State, Niger Delta, Nigeria: implication for groundwater management. Environ Monit Assess 191:617. https://doi.org/10.1007/s10661-019-7788-2
Pang H, Li Y, Yang J, Liang Y (2010) Effect of brackish water irrigation and straw mulching on soil salinity and crop yields under monsoonal climatic conditions. Agric Water Manag 97(12):1971–1977
Piper M (1944) A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union 25:914–928
Province G, Han Z, Tang C, Wu P (2015) Hydrogeochemical characteristics and associated mechanism based on groundwater dating in a karstic basin, Guizhou Province, China. Environ Earth Sci 73(1):67–76
Raghunath H (1987) Groundwater, 2nd edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Rajmohan RNN, Senthamilkumar UMS (2010) Evaluation of groundwater quality and its suitability for drinking and agricultural use in Thanjavur city, Tamil Nadu, India. Environ Monit Assess 171(1–4):289–308
Richards L (1954) Diagnosis and improvement of saline and alkali soil, Agriculture Handbook No: 60. United States Department of Agriculture, Washington
Saha P, Paul B (2019) Groundwater quality assessment in an industrial hotspot through interdisciplinary techniques. Environ Monit Assess 191(Suppl 2):326. https://doi.org/10.1007/s10661-019-7418-z
Sahraei H, Nozar P (2013) Geochemical evolution and quality assessment of water resources in the Sarcheshmeh copper mine area (Iran) using multivariate statistical techniques. Environ Earth Sci 69:1699–1718
Simsek C, Gunduz O (2007) IWQ index: a GIS-integrated technique to assess irrigation water quality IWQ index: a GIS-integrated technique to assess irrigation water quality. Environ Monit Assess 128:277–300
Simsek C, Elci A, Gunduz O, Erdogan B (2008) Hydrogeological and hydrogeochemical characterization of a karstic mountain region. Environ Geol 54(2):291–308. https://doi.org/10.1007/s00254-007-0817-4
Singh KR, Goswami AP, Kalamdhad AS, Kumar B (2019) Development of irrigation water quality index incorporating information entropy. Environ Dev Sustain. https://doi.org/10.1007/s10668-019-00338-z
Todd D (1980) Groundwater hydrology, 2nd edn. John Wiley & Sons, Hoboken
Wang S (2013) Groundwater quality and its suitability for drinking and agricultural use in the Yanqi Basin of Xinjiang Province, Northwest China. Environ Monit Assess 185:7469–7484
Wani AML (2019) Management of groundwater resources in karstic aquifer using visual modflow: case study of Altinova Turkey, MSc. Thesis, Akdeniz University, Environmental Engineering Department, Antalya, Turkey
WHO (2006) Guidelines for drinking-water quality, 4th edn. World Health Organisation, Geneva
Wilcox L (1955) Classification and use of irrigation waters. United States Department of Agriculture, Washington
Yilmaz I, Sayin C, Ozkan B (2005) Turkish greenhouse industry: Past, present, and future. New Zeal J Crop Hort 33(3):233–240
Zhang W, Ma L, Abuduwaii J, Ge Y, Issanova G, Saparov G (2019) Hydrochemical characteristics and irrigation suitability of surface water in the Syr Darya River, Kazakhstan. Environ Monit Assess 191:572. https://doi.org/10.1007/s10661-019-7713-8
Zouahri A, Dakak H, Douaik A, El Khadir M, Moussadek R (2015) Evaluation of groundwater suitability for irrigation in the Skhirat region, Northwest of Morocco. Environ Monit Assess 187:4184. https://doi.org/10.1007/s10661-014-4184-9
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research study was supported by the Scientific and Technological Research Council of Turkey (Project No.114Y696), Akdeniz University and State Hydraulic Works 13th Regional Directorate in Antalya, Turkey. The first author expresses sincere thanks to Akdeniz University for providing a conducive work environment also acknowledges the Turkish Scholarship (YTB) for their support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Alex Modi Lomoro Wani, Ziyad Abunada, Firdes Yenilmez, Ayse Muhammetoglu and Habib Muhammetoglu. The first draft of the manuscript was written by Alex Modi Lomoro Wani, Ziyad Abunada, Firdes Yenilmez, Ayse Muhammetoglu, Habib Muhammetoglu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors mutually agreed to publish the work in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wani, A.M.L., Abunada, Z., Yenilmez, F. et al. Comparative assessment of hydrochemical characterization and groundwater quality for irrigation in an autochthonous karst aquifer with the support of GIS: case study of Altinova, Turkey. Environ Earth Sci 83, 237 (2024). https://doi.org/10.1007/s12665-024-11548-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-024-11548-8