Abstract
Abdominal tuberculosis is an ancient problem with modern nuances in diagnosis and management. The two major forms are tuberculous peritonitis and gastrointestinal tuberculosis (GITB), while the less frequent forms are esophageal, gastroduodenal, pancreatic, hepatic, gallbladder and biliary tuberculosis. The clinicians need to discriminate the disease from the close mimics: peritoneal carcinomatosis closely mimics peritoneal tuberculosis, while Crohn’s disease closely mimics intestinal tuberculosis. Imaging modalities (ultrasound, computed tomography, magnetic resonance imaging and occasionally positron emission tomography) guide the line of evaluation. Research in diagnostics (imaging and endoscopy) has helped in the better acquisition of tissue for histological and microbiological tests. Although point-of-care polymerase chain reaction–based tests (e.g. Xpert Mtb/Rif) may provide a quick diagnosis, these have low sensitivity. In such situations, ancillary investigations such as ascitic adenosine deaminase and histological clues (granulomas, caseating necrosis, ulcers lined by histiocytes) may provide some specificity to the diagnosis. A diagnostic trial of antitubercular therapy (ATT) may be considered if all diagnostic armamentaria fail to clinch the diagnosis, especially in TB-endemic regions. Objective evaluation with clear endpoints of response is mandatory in such situations. Early mucosal response (healing of ulcers at two months) and resolution of ascites are objective criteria for early response assessment and should be sought at two months. Biomarkers, especially fecal calprotectin for intestinal tuberculosis, have also shown promise. For most forms of abdominal tuberculosis, six months of ATT is sufficient. Sequelae of GITB may require endoscopic balloon dilatation for intestinal strictures or surgical intervention for recurrent intestinal obstruction, perforation or massive bleeding.
Similar content being viewed by others
Introduction
Infection with Mycobacterium tuberculosis lasts a lifetime and the organism infects almost a quarter of the world population that remains at the risk of advancing to active disease [1, 2]. Tuberculosis affects nearly 10 million people and leads to death in more than a million people annually, despite being a preventable and curable disease [2]. It primarily involves the lung, but the incidence of extrapulmonary tuberculosis (EPTB) is around 15% globally. Abdominal TB is among the common sites of extrapulmonary involvement, where it tends to involve the gastrointestinal tract, peritoneum, lymph nodes and solid organs in that order. The diagnosis and management of abdominal TB are challenging: the disease is usually paucibacillary with a low yield of microbiological tests and it mimics many conditions closely, resulting in diagnostic confusion [3, 4]. In certain cases, relatively non-specific parameters such as ascitic fluid adenosine deaminase levels are utilized for diagnosis [5]. When the diagnosis remains unclear even after all these modalities, a therapeutic trial with antitubercular therapy (ATT) is often started in TB endemic regions and the response to the therapy is assessed. Since the disease is primarily a concern in the less developed world, the development of evidence-based diagnosis and treatment has lagged.
A recent survey of clinicians suggested significant variations in clinical practice with respect to the diagnostic modalities, treatment duration and follow-up modalities in abdominal TB [6]. Therefore, the present review aims at providing an evidence-based summary to inform clinical practice for abdominal TB. Because gastrointestinal tuberculosis (GITB, also termed intestinal TB) and tuberculous peritonitis (TBP, also termed peritoneal tuberculosis [PTB]) are the most frequent clinical problems, the review is largely focused on these two entities.
Methods
Although the present review is a narrative review, a search of two databases, Pubmed and Embase, was performed on December 1, 2022, to inform the review. We used MESH words and free terms to search for Abdominal Tuberculosis OR Peritoneal Tuberculosis OR Tuberculous peritonitis OR Gastrointestinal Tuberculosis OR Intestinal Tuberculosis. We aimed at providing evidence-based recommendations regarding several contentious areas in the diagnosis and management of abdominal TB, including evaluation pathways, diagnostic armamentarium to be used (standard culture or liquid culture, number/amount of tissue or fluid, which polymerase chain reaction (PCR)–based tests, whether to use adenosine deaminase and the cut-off for PTB) and treatment strategies (duration of ATT, additional role of ancillary therapies such as steroids, use of diagnostic trial and the duration of such a trial, appropriate methods of assessment after a diagnostic trial of ATT). Wherever systematic reviews (Tables 1 and 2) or randomized studies were available, we used these to summarize the management recommendations [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Where systematic reviews or randomized clinical trials (RCTs) were not available, we used observational studies to suggest an appropriate clinical approach. We also identify advances in the field that are likely to be useful in clinical practice in the coming times, lacunae in the current literature and avenues for future research.
Epidemiology of abdominal tuberculosis
The number of cases of abdominal TB as a fraction of all EPTB cases has been reported to vary from 2.7% to 21% [25, 26]. In a study from three states in India and based on the national tuberculosis program, abdominal TB constituted 12.8% of all EPTB cases [26]. Lower treatment completion rates and worse outcomes have been reported in abdominal TB [26, 27]. Among patients with abdominal TB, both GITB and tuberculous peritonitis have been reported as common sites [28, 29]. Because of a possible selection bias, PTB being easier to diagnose based on abdominal paracentesis, GITB is often reported as the commonest form of abdominal TB in most reports from tertiary care centers [25].
Certain comorbidities, especially chronic liver disease, specifically increase the predisposition to abdominal/PTB [30]. Additional risk factors for developing abdominal TB include younger age, female gender, Asian ethnicity, human immunodeficiency virus (HIV) coinfection, immunosuppression, diabetes mellitus and peritoneal dialysis [30,31,32].
Clinical presentation
In a systematic review on tuberculous peritonitis, abdominal pain (65%), fever (59%), weight loss (61%), diarrhea (21%) and constipation (11%) were the most frequently reported symptoms [32]. The presence of ascites (73%), abdominal tenderness (48%), hepatomegaly (28%) and splenomegaly (14%) were the most frequent clinical findings (16,197,489). Constitutional symptoms occur in half of the patients. Because of the non-specific nature of the symptoms, the diagnosis can be delayed (7–24 weeks from the onset of symptoms) [33]. Although less frequent than GITB, TBP may also have features of intestinal obstruction, especially in the presence of adhesions, peritoneal fibrosis or sclerosing encapsulating peritonitis (i.e. abdominal cocoon) [34].
The clinical presentation of intestinal TB is dominated by abdominal pain (30% to 88%), fever (21% to 73%), diarrhea (5% to 47%), loss of appetite (30% to 90%), loss of weight (8% to 80%), constipation (7% to 24%), and hematochezia (5% to 15%). Some patients may present with intestinal obstruction (3% to 36%) [35]. Concomitant or past pulmonary TB could be present in up to 25% of cases [36]. The rising incidence of inflammatory bowel disease (IBD) in India does not seem to have reduced the numbers of GITB. Hence distinguishing the two continues to be a major challenge [37].
Case definitions
A large number of case definitions and classifications for abdominal TB are in vogue and are based on clinical presentation, morphological patterns, basis of diagnosis and response to ATT (Fig. 1). Traditionally, Paustian criteria were proposed but are impractical in the modern era, as they rely on surgical specimens and animal inoculation. Logan proposed a response assessment that continues to be used to date for diagnosing GITB [38]. The cases of abdominal TB could be defined on the basis of clinical presentation—pain (strictures, hypertrophic lesions) or diarrhea (diffuse ulcers) for intestinal TB and pain predominant (peritoneal adhesions or fibrosis) or distension predominant (ascites in PTB) for tuberculous peritonitis [39]. The morphological patterns have also been described for intestinal TB and tuberculous peritonitis, but an overlap among these patterns is well recognized. A systematic review of definitions of PTB identified that the classification into wet, dry and fibrotic forms is dogged by overlapping features and is better avoided [39]. Therefore, clinicians should largely follow a case definition that provides information regarding the confidence of diagnosis—a hierarchical strategy may be helpful and would largely be consistent with the definitions of INDEX- TB guidelines [40]. A microbiologically positive case would have the highest confidence in the diagnosis, while in the absence of microbiological positivity, a diagnosis of a clinically diagnosed case is made. It is important to recognize that all clinically diagnosed cases of abdominal TB are not equal: certain findings such as caseating granulomas or high ascitic adenosine deaminase levels may provide more certainty, while a diagnosis based on consistent clinical-radiological findings and exclusion of alternative diagnosis is much less secure. The confidence in the diagnosis has clinical relevance because the clinicians would need to follow patients with possible abdominal TB more closely and evaluate them for objective evidence of response to ATT. Figure 1 suggests a hierarchical approach to the diagnosis of abdominal TB based on confidence in the diagnosis.
Evaluation
Serum markers/IGRA
Interferon-gamma release assays (IGRAs) have emerged as an important tool for the diagnosis of latent TB infection. These have also been used for possible discrimination of abdominal TB from mimics like Crohn’s disease or other causes of ascites. These are believed to be helpful as these are not impacted by Bacille Calmette-Guérin (BCG) vaccination.
In a meta-analysis of 12 studies, the diagnostic accuracy of peripheral blood (PB) T-SPOT and peritoneal fluid (PF) T-SPOT for TBP was evaluated. The pooled sensitivity and specificity of PB T-SPOT for diagnosing peritoneal TB were 91% and 78%, respectively, while the pooled positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were 4.05 and 0.13, respectively. On the other hand, the pooled sensitivity, specificity, PLR and NLR of PF T-SPOT for TBP were 90%, 78%, 6.35, and 0.14, respectively. The results summarized that both PB T-SPOT and PF T-SPOT are sensitive for diagnosing TBP. Still, the unsatisfactory specificity of these two methods limits application as rule-in tests for peritoneal TB diagnosis [13] (Table 1). In another systematic review, interferon-gamma levels in the ascitic fluid were reported to have an excellent sensitivity and specificity (93% and 99%, respectively), but the incremental values over ascitic ADA are uncertain [8].
IGRAs have also been evaluated in multiple studies as a diagnostic modality to discriminate GITB from Crohn’s disease (CD). Three meta-analyses have been published (Table 2) [19,20,21]. In the most recent meta-analysis by Xu et al., 12 studies were included and a pooled sensitivity of 82.8% (78.4–86.6%) and a pooled specificity of 86.7% (83.2–89.6%) were reported [21]. The authors of these meta-analyses concluded that IGRA is a good supplementary method to discriminate between intestinal tuberculosis (ITB) and CD. Some other studies, however, report poor sensitivity and specificity and question the utility of this test [41].
There are certain caveats to the use of this test—in TB-endemic regions, patients with IBD would also be exposed to tuberculosis. They may have a positive IGRA, while many with disseminated TB or malnutrition may not demonstrate immune responsiveness on exposure to TB antigens. A recent report highlighted the value of quantitative measurements using enzyme-linked immunoassay (ELISA), suggesting that the levels of > 100 pg/mL had a higher sensitivity and specificity for discriminating ITB from CD [42]. With the available evidence, the role of IGRA in the diagnosis of active abdominal TB is unclear and should not be routinely done in TB-endemic regions to diagnose active TB. However, it may have a role in excluding TB if a diagnosis of CD is considered in TB-endemic settings, with the caveats already mentioned.
Imaging modalities
The initial imaging used for evaluation is often abdominal ultrasound (USG) which can help in the evaluation of lymphadenopathy, peritoneal or omental thickening, ascites, mesenteric changes and bowel wall thickening. The use of bowel ultrasound in the evaluation of strictures has been reported in the setting of IBD, although this is yet to be reported systematically in the setting of GITB [43]. Nevertheless, ultrasound provides a good initial evaluation in suspected abdominal TB cases and can also provide material for microbiological, cytological or histopathological evaluation [44]. In a systematic review on the utility of abdominal ultrasonography for the diagnosis of TB in the setting of HIV, the sensitivity and specificity for the diagnosis of abdominal TB were 63% and 68%, respectively, against a microbiological standard. These findings suggest that a negative ultrasound should not be used to exclude abdominal TB in HIV-positive individuals [24].
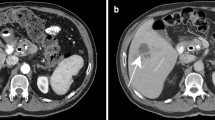
Computed tomography (CT) has emerged as an excellent tool for evaluating abdominal diseases because of its easy availability and excellent resolution. It provides good visualization of both luminal and extraluminal findings [43]. A systematic review of six studies compared the yield of 17 CT findings in discriminating TBP from peritoneal carcinomatosis. The highest diagnostic accuracy for TBP was achieved by smooth peritoneal thickening (AUC: 0.83), with fairly good specificity (84%), but limited sensitivity (59%). The location and presence of ascites showed poor diagnostic accuracy (AUC: 0.63) because of both poor sensitivity (50%) and specificity (58%). Lymph node necrosis and calcification showed an impressive specificity (95% and 100%, respectively) but poor sensitivity (10% and 12%, respectively) [7].
Another systematic review of six studies with 612 patients assessed the role of various CT findings in the discrimination of GITB and CD. Certain findings such as necrotic lymph nodes (sensitivity: 23% and specificity: 100%) and ileocecal involvement (sensitivity: 64% and specificity: 77%) suggested the diagnosis of GITB. Other features including comb sign, skip lesions, asymmetric bowel involvement, mural stratification, long segment involvement, fibrofatty proliferation and left-sided colonic involvement were associated with a diagnosis of CD. However, none of these findings (except for necrotic lymph nodes) were pathognomonic of a particular diagnosis [18]. Certain other CT findings, which could suggest a diagnosis of abdominal TB, include the presence of pulmonary involvement (15% to 25% cases), omental line or rim (thick uniformly enhancing outer rim of thickened omentum) and low visceral fat to subcutaneous fat ratio (< 0.63) [36, 45, 46]. Concomitant genitourinary involvement, especially in females with salpingitis, hydrosalpinx, adnexal lesions and tubo-ovarian masses, could suggest underlying TB [47]. USG or CT-guided fine-needle aspiration cytology or biopsy provides an opportunity to clinch a microbiological or cytological/histological diagnosis from extraintestinal lesions such as lymph nodes, peritoneal or omental deposits or thickening [44, 48].
Advances in MRI could further improve diagnostics and follow-up. Magnetic resonance enterography (MRE) has been shown to identify more strictures than barium studies [49]. Also, diffusion-weighted imaging and apparent diffusion coefficient (ADC) have been found to be helpful in assessing response as ADC values increase in responders [49, 50].
Ascitic tests
Typically, tubercular ascites is a low-serum ascites albumin gradient (SAAG) and high-protein ascites with a predominance of lymphocytes. However, this typical pattern may not be seen in patients with chronic liver disease (high SAAG), or malnutrition (low protein) [4]. Ascitic fluid cytology is routinely performed in patients with ascites and provides an opportunity to exclude important differential diagnoses, especially peritoneal carcinomatosis. Although typically lymphocyte dominance is seen in tubercular ascites, neutrophils could be predominant in early stages and peritoneal dialysis associated with TBP.
Ascitic adenosine deaminase
Adenosine deaminase (ADA) in peritoneal fluid offers a feasible, sensitive and highly specific test for TBP. While the multiple available test methods and different cut-offs by different studies can pose a challenge, as ascitic fluid tapping and analysis is easy to do and yields immediate results, ADA remains a relevant tool for screening and diagnosing PTB in India. Additionally, as PTB is a paucibacillary disease with low mycobacterial numbers, ADA has a special role given its sensitivity and specificity. Four meta-analyses have addressed the issue of diagnostic yield of ADA [9,10,11,12]. The most recent systematic review included 24 studies with 3,044 samples and found that the test had an excellent pooled sensitivity and specificity (93% and 95%, respectively) for the diagnosis of TBP. The PLR and NLR were also supportive of the use of this test. INDEX TB Guidelines suggest that a cut-off of 39 U/L be used for the diagnosis. However, there are certain caveats to the use of this test; false positives could occur in peritoneal carcinomatosis, lymphoma, hemorrhagic ascites and pus, while false negatives could occur in early stages or underlying cirrhosis (conflicting literature) [5, 51]. If the diagnosis of TBP is based solely on ascitic ADA, we usually perform paracentesis and cytological analyses three times to exclude peritoneal carcinomatosis with some degree of certainty [4, 5].
Microbiological tests on ascitic fluid
The yield of acid-fast bacilli (AFB) smear testing is extremely poor with a sensitivity of < 5%. We do not routinely perform AFB staining on ascitic fluid. Mycobacterial culture has a sensitivity of around 35%, but would be specific if positive [32]. There is a growing interest in PCR-based diagnostics, especially those which are available as point-of-care tools. Xpert Mtb/Rif has emerged as an important tool for microbiological diagnosis of pulmonary and some forms of extrapulmonary TB. The platform provides a point-of-care PCR-based diagnosis rapidly and safely. Additionally, it also provides information about rifampin resistance and helps in early diagnosis of multidrug-resistant tuberculosis (MDR-TB). Two systematic reviews have assessed the utility of Xpert Mtb/Rif testing for TBP [14, 15]. In one systematic review, 18 included studies and 1099 samples were included. The pooled sensitivity and specificity with respect to culture as a gold standard were 64% and 97%, respectively. However, against a comprehensive reference standard (eight studies, 643 samples), the pooled sensitivity and specificity were 30% and 100%. There are reports of other PCR-based tests, but these tests have not been validated. In a study of multiplex PCR (16SrRNA, IS6110, and devR-based primers), the sensitivity was 75.7% with a specificity of 100%, but the validation of this approach is required [52].
Morphological or visual appearance
Laparoscopy
Laparoscopy with peritoneal biopsy is a tool for rapid and accurate diagnosis of TBP. However, it is not commonly performed due to the invasive nature of the procedure, complications such as bowel perforation and bleeding and lack of availability at peripheral setups. Classical patterns of TBP on laparoscopy were described as peritoneal thickening with yellow-white tubercles and the visual appearance was reported to be diagnostic in a majority of cases [53]. Peritoneoscopy also provides an opportunity to sample the lesions and achieve a histological or microbiological confirmation.
Colonoscopy findings
Because the ileocecal region and the ascending colon are the most frequent sites of involvement in GITB, ileocolonoscopy is an important tool in diagnosing GITB. The procedure provides an opportunity to evaluate the morphological pattern of involvement and also obtain tissue for histological and microbiological evaluation. Although none of the endoscopic findings are specific to GITB, some findings are considered to be suggestive of GITB. In a systematic review published in abstract form by Du et al., 12 studies with 1134 patients were included. While the presence of transverse ulcers (sensitivity: 43% and specificity: 88%) and a patulous ileocecal valve (sensitivity: 38% and specificity: 91%) was suggestive of GITB, the presence of aphthous ulcers, cobblestone appearance, skip lesions and longitudinal ulcers was more suggestive of CD [54]. In a Bayesian meta-analysis that studied multiple parameters, these findings were confirmed [55].
Intestinal biopsy-based tests
Microbiology
The diagnosis of ITB often relies on testing of the intestinal tissue. It is important to obtain adequate tissue samples for evaluation. A study suggested that taking eight pieces (instead of four) increased the positivity of Mycobacterium Growth Indicator Tube-960 (MGIT) culture from 40% to 52.8% [56]. In real life, it may sometimes be difficult to obtain adequate tissue because samples are needed for multiple tests (histopathology, culture, polymerase chain reaction/Xpert Mtb/Rif). A recent standard treatment workflow by Indian Council of Medical Research (ICMR) suggested that at least six biopsy pieces be obtained in sterile saline for microbiological testing [54].
Microbiological positivity is the gold standard for the diagnosis of GITB. The tissue obtained by surgery/colonoscopy should be subjected to microbiological testing. Although AFB staining is extremely important for pulmonary samples, the positivity rates are extremely low for GITB. TB culture provides an important tool for the diagnosis of GITB and also helps in testing for drug sensitivity in appropriate clinical settings. Culture positivity has been reported to vary from 7% to 79%, but is usually less than 50% [35]. MGIT may be preferable to the traditional culture in the Lowenstein-Jensen medium [57]. This is because MGIT provides early detection and possibly has higher sensitivity.
In a systematic review of nine studies with 709 patients, the sensitivity and specificity of IS6110-based PCR testing for the diagnosis of GITB were 47% and 95%, respectively [22]. In a systematic review of five studies with 460 samples, the pooled sensitivity and specificity of Xpert Mtb/Rif for the diagnosis of GITB using intestinal tissue were 23% and 100%, respectively, as compared to a composite reference standard [15]. No reports are available on the utility of newer PCR-based diagnostics such as Xpert Ultra. One study has reported that the sensitivity and specificity of Truenat MTB Plus (TruPlus) were 70% and 100%, respectively [58]. As with peritoneal tuberculosis, multiplex PCR based on multiple probes has been found to be sensitive and specific but needs validation [52, 58].
Histopathology
Histopathological evaluation plays an important role in the diagnosis of GITB and its discrimination from CD. The findings on histology may include the presence of epithelioid cell granulomas, caseating necrosis, confluent granulomas and changes of chronic inflammation [59]. Since GITB is usually a paucibacillary disease, clinicians may have to rely on histology for the diagnosis. In a systematic review of diagnostic accuracy, including 10 studies with 692 patients, three histological features were found to have high specificity for the diagnosis of GITB (as compared to CD). These included caseating granuloma (sensitivity of 0.21 and specificity of 100%), confluent granuloma (sensitivity of 0.38 and specificity of 99%) and ulcers lined by histiocytes (sensitivity of 0.41 and specificity of 94%) [23]. These three features, although specific, have poor sensitivity and none of these is positive in half of the cases of GITB. Therefore, a conclusive histopathological diagnosis is only possible in some cases. Further, granulomas in GITB are predominantly submucosal in location and are dense (> 5/high-power field [HPF]) and relatively larger in size (macrogranuloma, > 200 µm) [55, 60]. The presence of lymph nodal granuloma in the absence of intestinal inflammation is highly specific for TB. Granulomas in CD are commonly smaller in size (microgranuloma, < 200 µm), discrete and fewer in number [60].
Table 3 provides the sensitivity of various modalities for the diagnosis of TBP and GITB.
Treatment and response
Antitubercular therapy and duration
A Cochrane meta-analysis of three RCTs (328 participants) compared the six-month regimen with the nine-month regimen of ATT to treat adults with abdominal TB. The relapse rates were not higher in patients who received a shorter duration (six months) of therapy with isoniazid, rifampin, pyrazinamide and ethambutol. Also, the clinical cure rates at the end of therapy were similar between the two groups [17]. Out of the three RCTs, only one included participants with PTB. The participants in this trial were treated thrice weekly under a directly observed therapy program and followed up for 12 months after completing ATT. No difference was reported between the six-month and nine-month regimen on per-protocol analysis (91.5% vs. 90.8%) or intent-to-treat analysis (75% vs. 75.85%) [59]. Additional observational data suggest that six months of therapy is sufficient in most cases, although the guidelines provide clinicians with an option of extending the duration of therapy on a case-to-case basis [28].
Role of steroids
Corticosteroids could potentially offer benefits when used as an adjunctive by reducing inflammation and preventing post-inflammatory fibrosis and are used in TB meningitis and pericardial TB. A meta-analysis evaluating the use of steroids for PTB showed adjunctive steroids used with ATT were more effective when compared with using ATT for the prevention of composite endpoint, symptomatic stricture and intestinal obstruction. However, the study noted that due to the poor quality of studies involved in the review and meta-analysis, the findings could not be generalized and there is a need for prospective well-controlled trials [16]. Therefore, routine use of steroids is not recommended for abdominal TB.
Endoscopic interventions and surgery
Stricturing disease is common in GITB and may occur in a quarter of patients with GITB. While three-fourths of the stricturing disease have a clinical response to ATT (unpublished meta-analysis), many patients continue to be symptomatic even after ATT and require additional therapy (endoscopic dilatation or surgery) [61, 62]. Further, many patients may present directly with intestinal obstruction and may need emergency surgery. In patients with ongoing symptoms in spite of ATT, endoscopic dilatation could be done for endoscopically reachable strictures [62]. The endpoint of dilatation is not well defined, but symptom resolution is aimed for. Usually, endoscopic dilatation is safe and efficacious [62]. Indirect evidence from CD-related strictures suggests a dilatation of 15–18 mm and passability of a standard colonoscope as additional criteria, but these have not been validated in the setting of GITB [35]. Strictures in GITB are likely to behave differently from CD-related strictures because effective ATT would ensure the absence of ongoing inflammation and therefore, the effects of dilatation may be more lasting. Apart from through-the-scope (TTS) balloon dilatation, for which data is available, there is no data for additional modalities, including endoscopic stricturotomy, in GITB. Certain patients may require surgery, especially if they are suffering from unrelenting intestinal obstruction, recurrent abdominal pain or episodes of obstruction, massive gastrointestinal bleeding or perforation peritonitis [63, 64].
Response assessment
As mentioned earlier, the diagnosis of abdominal TB is difficult because of the low positivity of microbiological tests. Certain diseases may closely mimic abdominal TB—like peritoneal carcinomatosis (mimics TBP) and CD (mimics GITB). Unfortunately, clinical responses to ATT can be misleading. There is evidence to suggest that antimycobacterial therapy may result in clinical response in patients with CD [65, 66]. On the other hand, patients with GITB may continue to be symptomatic due to underlying strictures. In cases where the initial diagnosis was uncertain, it is important to look for objective evidence of response to ATT. Trial of ATT (variably termed as a diagnostic trial or a therapeutic trial) is the standard strategy to discriminate GITB and CD in TB-endemic areas. For ITB, healing of ulcers with ATT is an objective criterion of response to ATT, while the resolution of ascites is the criterion in PTB [66, 67]. Because the delay in diagnosis of CD due to ATT could potentially result in worse outcomes, it is important to make the discrimination between the two entities as early as possible. In a study of > 700 patients with CD, ATT was responsible for a diagnostic delay and could result in clinical response even in CD. A diagnostic delay was associated with more stenosing complications and the need for surgery [68]. In another report, the progression of an inflammatory pattern of CD to stricturing disease was reported with the administration of ATT [69].
In this regard, multiple studies have suggested that objective evidence of mucosal healing can be seen early (at two months) and could potentially reduce unduly prolonged ATT [70, 71]. Also, a two-month colonoscopy to look for early mucosal response provides an opportunity to identify and address the causes for lack of response, including alternative diagnosis or drug resistance [70]. In patients who are not willing to undergo colonoscopy, biomarkers such as fecal calprotectin can be used as a surrogate marker of mucosal response [72, 73]. Fecal calprotectin appears to be a better biomarker as compared to serum C-reactive protein (CRP) measurements [72]. The utility of these non-invasive parameters for follow-up was demonstrated in a study performed during the coronavirus disease - 19 (COVID-19) pandemic, when physical follow-ups were difficult and endoscopic procedures restricted [74].
Follow-up in other types of abdominal TB utilizes a combination of imaging and clinical follow-up [75]. Tubercular abdominal cocoon could respond clinically to ATT, but a significant number will require surgery [76].
Drug resistance
Drug resistance is an important global concern with regard to TB. Drug resistance in abdominal TB is likely to be similar to overall drug resistance in a particular region. Several studies have reported drug resistance in GITB. In a culture-based study from Western India, of the 43 patients with culture positivity, 23% had resistance to at least one first-line drug, while 14% had MDR-TB [77]. In a recent study on 30 ileocecal biopsies with positive TB cultures, four each (13.3%) had isoniazid and MDR, while two each (6.7%) were pre-extremely drug resistant (XDR) and mono-fluoroquinolone resistant [78]. This finding needs to be interpreted with caution, as only a subset of patients had positive cultures. Another study from the Western India reported an MDR-TB rate of 11.5% [79]. The rates were much lower (4%) in an Xpert Mtb/Rif-based study from Northern India [80].
Figure 2 summarizes an evidence-based approach to the evaluation and treatment of abdominal TB and is largely consistent with ICMR standard treatment workflow.
Additional types of abdominal tuberculosis
Gastroduodenal tuberculosis
The stomach is an uncommon site of TB but could present with symptoms such as epigastric pain, gastric outlet obstruction, hematemesis and failure to thrive (in children) along with constitutional symptoms. Endoscopic findings can include ulcers, mass or growth, nodularity, stricture or extrinsic compression [81]. Deeper biopsies using endoscopic mucosal resection or well technique (biopsy upon biopsy) may improve the diagnostic yield. Endoscopic ultrasound could help in targeting extraluminal lesions, including the lymph nodes. Gastric cancer is an important differential diagnosis. Endoscopic balloon dilatation (usually successful) or surgery may be needed if obstructive symptoms do not improve with ATT [81, 82].
Pancreatic tuberculosis
Pancreatic TB is another uncommon manifestation of TB that closely mimics pancreatic cancer. It usually presents as a solid pancreatic mass (especially in the head of the pancreas), but may present as a cystic lesion or peripancreatic lymphadenopathy [83]. The clinical manifestations may include abdominal pain, jaundice and constitutional symptoms. Although it was usually diagnosed as a histological surprise for surgical resection of presumed pancreatic cancer, the advent of endoscopic ultrasound has resulted in more cases being diagnosed as part of the evaluation of pancreatic masses. It should always be considered in the differential diagnosis of pancreatic masses in TB-endemic regions [84].
Hepatobiliary tuberculosis
Hepatic involvement in TB could occur as part of localized disease (mass or tuberculoma or abscess) or systemic disease (granulomatous hepatitis). The clinical presentation could be due to systemic disease (fever, weight loss, organomegaly) or local disease (abdominal pain or tenderness) [85]. Liver biopsy has an excellent yield in granulomatous hepatitis, while image-guided fine-needle aspiration biopsy can help achieve diagnosis in localized forms of the disease. Microbiological yield from hepatic lesions is usually good [86]. Hepatic tuberculosis should be considered in non-resolving liver abscess, hepatic space-occupying lesion(s) or infiltrative pattern of liver function tests with predominant alkaline phosphatase elevations. Biliary involvement could also occur in the form of biliary strictures or lymph nodal compression of the biliary system [85]. Gallbladder TB is very rare and mimics gallbladder cancer [86, 87].
Future aspects
The low sensitivity of microbiological tests is the Achilles heel in the diagnosis of abdominal TB. It is unclear if increasing the amount of tissue or ascitic fluid could increase the mycobacterial yield on culture or other microbiological tests. It also remains to be seen if improvement in PCR-based diagnostics could improve the diagnostic yield. The use of newer diagnostic tests such as Xpert Ultra, although reported to have better sensitivity for the diagnosis of tuberculous pleural effusions, has not been reported for TBP [88]. Xpert XDR could also help in the detection of drug resistance to second-line therapies but has not been tested in abdominal TB. Multiplex PCRs which have shown to be of excellent sensitivity and specificity need to be validated in multicenter prospective studies before the clinical application can become routine.
The evaluation of small-bowel TB has been difficult because of the difficulty in accessing this region. Capsule endoscopy could help visualize the lesions but is limited by the inability to sample the lesions [89]. Advancements in small-bowel endoscopy including motorized spiral endoscopy could help improve the diagnosis of small-bowel TB. The other interest has been in using biomarkers including tumor markers (to discriminate TBP from peritoneal carcinomatosis), cytokines, CD4 + CD25 + FOXP3 + T-regulatory cells > 31.3% in peripheral blood (to discriminate GITB and CD), proteomic-based approaches, and nuclear medicine, including fluorodeoxyglucose (FDG)-positron emission tomography (PET) CT [90,91,92,93,94]. While some of these approaches have not been helpful, others, like the use of T-regulatory cells, appear promising but need validation at additional centers and assessment of feasibility before routine application. Some reports have evaluated the use of artificial intelligence, including its application on textual data (reports) or radiological images with fair discriminative power, but these are, as yet, beyond the realm of clinical use. Multiparameter models have also been assessed, and a model based on a Bayesian network meta-analysis seems to perform better than other models and is available online to discriminate ITB and CD [55].
Abdominal TB continues to be an important concern in many regions of the globe. The rising incidence of IBD in these regions poses additional challenges for diagnosis and management. Low diagnostic yield of microbiological tests in ascitic fluid and tissue biopsies is a concern and necessitates a diagnostic trial of ATT in a subset of patients. Close follow-up with objective evidence of response to ATT is essential in such cases.
References
Barberis I, Bragazzi NL, Galluzzo L, Martini M. The history of tuberculosis: from the first historical records to the isolation of Koch’s bacillus. J Prev Med Hyg. 2017;58:E9–12.
Global tuberculosis report 2022. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. Accessed from https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 1st February, 2022
Sharma V. Differentiating intestinal tuberculosis and Crohn disease: quo vadis. Expert Rev Gastroenterol Hepatol. 2020;14:647–50.
Sharma V, Debi U, Mandavdhare HS, Prasad KK. Tuberculosis and other mycobacterial infections of the abdomen. In: Kuipers EJ, editors. Encyclopedia of Gastroenterology. 2nd ed. Academic Press; 2020. p. 646–659.
Dahale AS, Dalal A. Evidence-based commentary: ascitic adenosine deaminase in the diagnosis of peritoneal tuberculosis. J Gastrointest Infect. 2022;12:57–60.
Dawra S, Sharma V, Sharma A, Dutta U, Chawla MPS, Kochhar R. Survey of practices in managing abdominal TB. Int J Tuberc Lung Dis. 2020;24:1109–11.
Chen J, Liu S, Tang Y, et al. Diagnostic performance of CT for differentiating peritoneal tuberculosis from peritoneal carcinomatosis: a systematic review and meta-analysis. Clin Radiol. 2020;75:396.e7-396.
Su SB, Qin SY, Guo XY, Luo W, Jiang HX. Assessment by meta-analysis of interferon-gamma for the diagnosis of tuberculous peritonitis. World J Gastroenterol. 2013;19:1645–51.
Riquelme A, Calvo M, Salech F, et al. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705–10.
Shen YC, Wang T, Chen L, et al. Diagnostic accuracy of adenosine deaminase for tuberculous peritonitis: a meta-analysis. Arch Med Sci. 2013;9:601–7.
Tao L, Ning HJ, Nie HM, Guo XY, Qin SY, Jiang HX. Diagnostic value of adenosine deaminase in ascites for tuberculosis ascites: a meta-analysis. Diagn Microbiol Infect Dis. 2014;79:102–7.
Zhou R, Qiu X, Ying J, et al. Diagnostic performance of adenosine deaminase for abdominal tuberculosis: A systematic review and meta-analysis. Front Public Health. 2022;10:938544.
Luo Y, Xue Y, Mao L, et al. Diagnostic value of T-SPOT.TB assay for tuberculous peritonitis: A meta-analysis. Front Med (Lausanne). 2020;7:585180.
Kohli M, Schiller I, Dendukuri N, et al. Xpert MTB/RIF Ultra and Xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2021;1:CD012768.
Sharma V, Soni H, Kumar-M P, et al. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti Infect Ther. 2021;19:253–65.
Soni H, Bellam BL, Rao RK, et al. Use of steroids for abdominal tuberculosis: a systematic review and meta-analysis. Infection. 2019;47:387–94.
Jullien S, Jain S, Ryan H, Ahuja V. Six-month therapy for abdominal tuberculosis. Cochrane Database Syst Rev. 2016;11:CD012163.
Kedia S, Sharma R, Sreenivas V, et al. Accuracy of computed tomographic features in differentiating intestinal tuberculosis from Crohn’s disease: a systematic review with meta-analysis. Intest Res. 2017;15:149–59.
Chen W, Fan JH, Luo W, Peng P, Su SB. Effectiveness of interferon-gamma release assays for differentiating intestinal tuberculosis from Crohn’s disease: a meta-analysis. World J Gastroenterol. 2013;19:8133–40.
Ng SC, Hirai HW, Tsoi KK, et al. Systematic review with meta-analysis: accuracy of interferon-gamma releasing assay and anti-Saccharomyces cerevisiae antibody in differentiating intestinal tuberculosis from Crohn’s disease in Asians. J Gastroenterol Hepatol. 2014;29:1664–70.
Xu H, Li Y, Qian JM. A meta-analysis of the accuracy of interferon-γ release assays in differentiating intestinal tuberculosis from Crohn’s disease in Asia. Zhonghua Nei Ke Za Zhi. 2016;55:535–40.
Jin T, Fei B, Zhang Y, He X. The diagnostic value of polymerase chain reaction for Mycobacterium tuberculosis to distinguish intestinal tuberculosis from Crohn’s disease: a meta-analysis. Saudi J Gastroenterol. 2017;23:3–10.
Du J, Ma YY, Xiang H, Li YM. Confluent granulomas and ulcers lined by epithelioid histiocytes: new ideal method for differentiation of ITB and CD? A meta analysis. PLoS One. 2014;9:e103303.
Van Hoving DJ, Griesel R, Meintjes G, Takwoingi Y, Maartens G, Ochodo EA. Abdominal ultrasound for diagnosing abdominal tuberculosis or disseminated tuberculosis with abdominal involvement in HIV-positive individuals. Cochrane Database Syst Rev. 2019;9:CD012777.
Nath P. Epidemiology of gastrointestinal tuberculosis. In: Sharma V, editor. Tuberculosis of the gastrointestinal system. Singapore: Springer; 2022. https://doi.org/10.1007/978-981-16-9053-2_2.
Cherian JJ, Lobo I, Sukhlecha A, et al. Treatment outcome of extrapulmonary tuberculosis under Revised National Tuberculosis Control Programme. Indian J Tuberc. 2017;64:104–8.
Ade S, Harries AD, Trébucq A, et al. National profile and treatment outcomes of patients with extrapulmonary tuberculosis in Bénin. PLoS One. 2014;9:e95603.
Mandavdhare HS, Singh H, Dutta U, Sharma V. A real-world experience with 6 months of antitubercular therapy in abdominal tuberculosis. JGH Open. 2019;3:201–5.
Singh A, Sahu MK, Panigrahi M, et al. Abdominal tuberculosis in Indians: Still very pertinent. J Clin Tuberc Other Mycobact Dis. 2019;15:100097.
Jeong YJ, Kang JY, Kim HW, et al. Association of underlying comorbidities and sites of tuberculosis: an analysis using surveillance data. BMC Pulm Med. 2022;22:417.
Al-Zanbagi AB, Shariff MK. Gastrointestinal tuberculosis: a systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol. 2021;27:261–74.
Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis–presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685–700.
Shariff MK, Alzanbagi A, Sanai FM. Peritoneal tuberculosis. In: Sharma V, ed. Tuberculosis of the Gastrointestinal System. Singapore: Springer; 2022. https://doi.org/10.1007/978-981-16-9053-2_10.
Sharma V, Singh H, Mandavdhare HS. Tubercular abdominal cocoon: systematic review of an uncommon form of tuberculosis. Surg Infect (Larchmt). 2017;18:736–41.
Kedia S, Ahuja V. Intestinal tuberculosis: an overview. In: Sharma V, ed. Tuberculosis of the Gastrointestinal System. Singapore: Springer; 2022. https://doi.org/10.1007/978-981-16-9053-2_6.
Kedia S, Sharma R, Vuyyuru SK, et al. Addition of computed tomography chest increases the diagnosis rate in patients with suspected intestinal tuberculosis. Intest Res. 2022;20:184–91.
Dhoble P, Desai D, Abraham P. Is the rise in Crohn’s disease in India accompanied by a fall in intestinal tuberculosis? A single-center experience Indian J Tuberc. 2021;68:210–4.
Logan VS. Anorectal tuberculosis. Proc R Soc Med. 1969;62:1227–30.
Ahamed ZR, Shah J, Agarwala R, et al. Controversies in classification of peritoneal tuberculosis and a proposal for clinico-radiological classification. Expert Rev Anti Infect Ther. 2019;17:547–55.
Sharma SK, Ryan H, Khaparde S, et al. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017;145:448–63.
Sachdeva K, Kumar P, Kante B, et al. Interferon-gamma release assay has poor diagnostic accuracy in differentiating intestinal tuberculosis from Crohn’s disease in tuberculosis endemic areas. Intest Res. 2022. https://doi.org/10.5217/ir.2022.00010.
Zhao Y, Xu M, Chen L, Liu Z, Sun X. Levels of TB-IGRA may help to differentiate between intestinal tuberculosis and Crohn’s disease in patients with positive results. Therap Adv Gastroenterol. 2020;18:1756284820922003.
Goyal P, Shah J, Gupta S, Gupta P, Sharma V. Imaging in discriminating intestinal tuberculosis and Crohn’s disease: past, present and the future. Expert Rev Gastroenterol Hepatol. 2019;13:995–1007.
Kumar S, Gupta P, Sharma V, et al. Role of ultrasound-guided fine-needle aspiration cytology of omentum in diagnosis of abdominal tuberculosis. Surg Infect (Larchmt). 2019;20:91–4.
Ramanan RV, Venu V. Differentiation of peritoneal tuberculosis from peritoneal carcinomatosis by the omental rim sign. A new sign on contrast enhanced multidetector computed tomography. Eur J Radiol. 2019;113:124–34.
Yadav DP, Madhusudhan KS, Kedia S, et al. Development and validation of visceral fat quantification as a surrogate marker for differentiation of Crohn’s disease and intestinal tuberculosis. J Gastroenterol Hepatol. 2017;32:420–6.
Naeem M, Zulfiqar M, Siddiqui MA, et al. Menias CO. Imaging manifestations of genitourinary tuberculosis Radiographics. 2021;41:1123–43.
Gupta P, Kumar S, Sharma V, et al. Common and uncommon imaging features of abdominal tuberculosis. J Med Imaging Radiat Oncol. 2019;63:329–39.
Krishna S, Kalra N, Singh P, et al. Small-bowel tuberculosis: a comparative study of MR enterography and small-bowel follow-through. AJR Am J Roentgenol. 2016;207:571–7.
Mathur P, Sharma R, Kandasamy D, Kedia S, Gamanagatti S, Ahuja V. Can ADC be used as a surrogate marker of response to therapy in intestinal tuberculosis? Abdom Radiol (NY). 2019;44:3006–18.
Dahale AS, Puri AS, Sachdeva S, et al. Reappraisal of the role of ascitic fluid adenosine deaminase for the diagnosis of peritoneal tuberculosis in cirrhosis. Korean J Gastroenterol. 2021;78:168–76.
Hallur V, Sharma M, Sethi S, et al. Development and evaluation of multiplex PCR in rapid diagnosis of abdominal tuberculosis. Diagn Microbiol Infect Dis. 2013;76:51–5.
Bhargava DK, Shriniwas, Chopra P, Nijhawan S, Dasarathy S, Kushwaha AK. Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol. 1992;87:109–12.
Du J, Ma YY, Ma PP, Chen CX. Endoscopic features for differentiation between Crohn’s disease (CD) and intestinal tuberculosis (ITB): a meta-analysis. J Dig Dis 2014; 15 Suppl. 1: 147.
Limsrivilai J, Shreiner AB, Pongpaibul A, et al. Meta-analytic Bayesian model for differentiating intestinal tuberculosis from Crohn’s disease. Am J Gastroenterol. 2017;112:415–27.
Mehta V, Desai D, Abraham P, et al. Do additional colonoscopic biopsies increase the yield of Mycobacterium tuberculosis culture in suspected ileo-colonic tuberculosis? Indian J Gastroenterol. 2018;37:226–30.
ADULT ABDOMINAL TUBERCULOSIS. Standard Treatment Workflows of India, 2022, Special Edition on Paediatric and Extrapulmonary Tuberculosis, Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India Accessed https://stw.icmr.org.in/images/Adult_Extr_Tuberculosis/1_Adult_Abdominal_TB_18032022.pdf on 1st February 2023
Sharma K, Sharma M, Sharma V, et al. Evaluating diagnostic performance of Truenat MTB Plus for gastrointestinal tuberculosis. J Gastroenterol Hepatol. 2022;37(8):1571–8.
Makharia GK, Ghoshal UC, Ramakrishna BS, et al. Intermittent directly observed therapy for abdominal tuberculosis: a multicenter randomized controlled trial comparing 6 months versus 9 months of therapy. Clin Infect Dis. 2015;61:750–7.
Pulimood AB, Ramakrishna BS, Kurian G, et al. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn’s disease from tuberculosis. Gut. 1999;45:537–41.
Aggarwal P, Kedia S, Sharma R, et al. Tubercular intestinal strictures show a poor response to anti-tuberculous therapy. Dig Dis Sci. 2017;62:2847–56.
Kumar P, Jena A, Birda CL, et al. Safety and efficacy of non-fluoroscopic endoscopic dilatation of gastrointestinal tuberculosis related strictures. BMC Gastroenterol. 2022;22:60.
Singh H, Krishnamurthy G, Rajendran J, et al. Surgery for abdominal tuberculosis in the present era: experience from a tertiary-care center. Surg Infect (Larchmt). 2018;19:640–5.
Limpin ET, Lopez MPJ, Maglangit SACA, et al. Surgical management of patients with GI tuberculosis. Dis Colon Rectum. 2023;66:106–12.
Agrawal G, Clancy A, Sharma R, Huynh R, Ramrakha S, Borody T. Targeted combination antibiotic therapy induces remission in treatment-naïve Crohn’s disease: a case series. Microorganisms. 2020;8:371.
Pratap Mouli V, Munot K, Ananthakrishnan A, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn’s disease. Aliment Pharmacol Ther. 2017;45:27–36.
Sharma V, Mandavdhare HS, Lamoria S, Singh H, Kumar A. Serial C-reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig Liver Dis. 2018;50:559–62.
Banerjee R, Pal P, Girish BG, Reddy DN. Risk factors for diagnostic delay in Crohn's disease and their impact on long-term complications: how do they differ in a tuberculosis endemic region? Aliment Pharmacol Ther. 2018;47:1367–74.
Gupta A, Pratap Mouli V, Mohta S, et al. Antitubercular therapy given to differentiate Crohn’s disease from intestinal tuberculosis predisposes to stricture formation. J Crohns Colitis. 2020;14:1611–8.
Sharma V, Mandavdhare HS, Dutta U. Letter: mucosal response in discriminating intestinal tuberculosis from Crohn’s disease-when to look for it? Aliment Pharmacol Ther. 2018;47:859–60.
Park YS, Jun DW, Kim SH, et al. Colonoscopy evaluation after short-term anti-tuberculosis treatment in nonspecific ulcers on the ileocecal area. World J Gastroenterol. 2008;14:5051–8.
Sharma V, Verma S, Kumar-M P, et al. Serial measurements of faecal calprotectin may discriminate intestinal tuberculosis and Crohn’s disease in patients started on antitubercular therapy. Eur J Gastroenterol Hepatol. 2021;33:334–8.
Jo HH, Kim EY, Jung JT, et al. Value of fecal calprotectin measurement during the initial period of therapeutic anti-tubercular trial. Clin Endosc. 2022;55:256–62.
Shukla J, Jena A, Singh H, Mandavdhare HS, Dutta U, Sharma V. Management of gastrointestinal tuberculosis during COVID pandemic: lessons for posterity. Dig Liver Dis. 2021;53:394–6.
Sharma V, Singh H, Mandavdhare HS. Defining ‘satisfactory response’ to therapy in abdominal tuberculosis: a work in progress. Infect Disord Drug Targets. 2020;20:111–4.
Sharma V, Mandavdhare HS, Rana SS, Singh H, Kumar A, Gupta R. Role of conservative management in tubercular abdominal cocoon: a case series. Infection. 2017;45:601–6.
Sonambekar A, Desai D, Abraham P, et al. Drug resistance in intestinal tuberculosis: a reason to worry? JGH Open. 2017;1:22–4.
Sharma K, Sharma M, Sharma V, et al. MTBDRplus and MTBDRsl for simultaneous diagnosis of gastrointestinal tuberculosis and detection of first-line and second-line drug resistance. J Gastroenterol Hepatol. 2023. https://doi.org/10.1111/jgh.16124.
Udgirkar S, Jain S, Pawar S, Chandnani S, Contractor Q, Rathi P. Clinical profile, drug resistance pattern and treatment outcomes of abdominal tuberculosis patients in Western India. Arq Gastroenterol. 2019;56:178–83.
Bellam BL, Mandavdhare HS, Sharma K, et al. Utility of tissue Xpert-Mtb/Rif for the diagnosis of intestinal tuberculosis in patients with ileocolonic ulcers. Ther Adv Infect Dis. 2019;6:2049936119863939.
Shah J, Maity P, Kumar-M P, Jena A, Gupta P, Sharma V. Gastroduodenal tuberculosis: a case series and a management focused systematic review. Expert Rev Gastroenterol Hepatol. 2021;15:81–90.
Dalal A, Puri AS, Sachdeva S, Sakuja P. Nonsurgical management of gastroduodenal tuberculosis: Nine-year experience from a tertiary referral center. Endosc Int Open. 2019;7:E1248-52.
Sharma V, Rana SS, Kumar A, Bhasin DK. Pancreatic tuberculosis. J Gastroenterol Hepatol. 2016;31:310–8.
Panic N, Maetzel H, Bulajic M, Radovanovic M, Löhr JM. Pancreatic tuberculosis: a systematic review of symptoms, diagnosis and treatment. United European Gastroenterol J. 2020;8:396–402.
Amarapurkar DN, Patel ND, Amarapurkar AD. Hepatobiliary tuberculosis in western India. Indian J Pathol Microbiol. 2008;51:175–81.
Hickey AJ, Gounder L, Moosa MY, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infect Dis. 2015;15:209.
Krishnamurthy G, Singh H, Rajendran J, et al. Gallbladder tuberculosis camouflaging as gallbladder cancer - case series and review focusing on treatment. Ther Adv Infect Dis. 2016;3:152–7.
Aggarwal AN, Agarwal R, Dhooria S, Prasad KT, Sehgal IS, Muthu V. Xpert MTB/RIF Ultra versus Xpert MTB/RIF for diagnosis of tuberculous pleural effusion: a systematic review and comparative meta-analysis. PLoS One. 2022;17: e0268483.
Rana SS, Sharma V, Sharma R, Nada R, Gupta R, Bhasin DK. Capsule endoscopy in small bowel Crohn’s disease and tuberculosis. Trop Doct. 2017;47:113–8.
Jain T, Ram S, Kumar H, Saroch A, Sharma V, Singh H. Ascitic and serum levels of tumor biomarkers (CA 72–4, CA 19–9, CEA and CA 125) in discrimination of cause of ascites: a prospective study. Arq Gastroenterol. 2022;59:198–203.
Gupta A, Sharma K, Sharma V, et al. Comparative evaluation of interleukin-10, transforming growth factor-β, and interleukin-17 in gastrointestinal tuberculosis and Crohn’s disease. Int J Mycobacteriol. 2022;11:384–8.
Rampal R, Kedia S, Wari MN, et al. Prospective validation of CD4+CD25+FOXP3+ T-regulatory cells as an immunological marker to differentiate intestinal tuberculosis from Crohn’s disease. Intest Res. 2021;19:232–8.
Rukmangadachar LA, Makharia GK, Mishra A, et al. Proteome analysis of the macroscopically affected colonic mucosa of Crohn's disease and intestinal tuberculosis. Sci Rep. 2016;6:23162.
Singh AK, Kumar R, Gupta P, et al. FDG-PET-CT enterography helps determine clinical significance of suspected ileocecal thickening: a prospective study. Dig Dis Sci. 2021;66:1620–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DKJ, MMP, and VS declare no competing interests.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way is the Honorary Editor-in-Chief, Editorial Board members, the Indian Society of Gastroenterology or printer/publishers responsible for the results/findings and content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jha, D.K., Pathiyil, M.M. & Sharma, V. Evidence-based approach to diagnosis and management of abdominal tuberculosis. Indian J Gastroenterol 42, 17–31 (2023). https://doi.org/10.1007/s12664-023-01343-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-023-01343-x