Abstract
Background
Acute kidney injury (AKI) heralds deterioration in patients with decompensated chronic liver disease (DCLD). Serum creatinine (sCr), a component of the model for end-stage liver disease-sodium (MELD-Na) prognostic score, has limitations in patients with DCLD. We evaluated the prognostic role of urine neutrophil gelatinase-associated lipocalin (NGAL) in DCLD and its ability to sub-type AKI.
Methods
Total 147 consecutive patients hospitalized between June 2018 and June 2020 for complications of DCLD were evaluated. Urine NGAL was estimated and demographic, clinical and biochemical parameters recorded at baseline. Participants were followed up till the end of study period or mortality, whichever came earlier. Primary outcomes included all-cause mortality and time to death after index hospitalization. Secondary outcomes included the presence and type of AKI, need for intensive care unit (ICU) stay, length of ICU/hospital stay, in-hospital mortality, development of new-onset/recurrent AKI and recurrent hospitalization after index admission.
Results
Urine NGAL was highest in acute tubular necrosis (ATN), lowest in pre-renal azotemia (PRA) and intermediate in hepatorenal syndrome (HRS-AKI). Urine NGAL (p = 0.0208) was superior to sCr (p = 0.0388) and inferior to fractionated excretion of sodium (FENa) (p = 0.0013) in stratifying AKI. A cut-off of 203.9 ng/mL discriminated between HRS and PRA with sensitivity 77.8% and specificity 68.7%. Urine NGAL correlated with MELD-Na score, need for ICU stay, in-hospital mortality and mortality at three and six months. Two-year survival was significantly lower in patients with urine NGAL > 205 ng/mL. Addition of log-urine-NGAL score did not improve predictive performance of MELD-Na.
Conclusion
Urine NGAL could identify AKI sub-types and correlated with short-term clinical outcomes, including mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) is a frequently encountered complication in patients with decompensated chronic liver disease (DCLD) with an estimated prevalence of 20% to 50% among hospitalized patients [1, 2]. AKI in DCLD has a wide spectrum ranging from pre-renal azotemia (PRA) to hepatorenal syndrome (HRS) and intrinsic AKI or acute tubular necrosis (ATN) [3]. The traditional concept of HRS as a purely ‘functional’ form of renal impairment was challenged by the advent of novel urinary biomarkers of AKI, which have connoted an additional ‘structural’ component to pathophysiology of HRS [4, 5]. The onset of AKI often heralds a downhill course in the natural history of decompensated cirrhosis and its early diagnosis and management are imperative to improving clinical outcomes.
Serum creatinine (sCr), despite its limitations as a biomarker of AKI in DCLD, still forms the fulcrum on which all standard definitions of AKI and prognostic scores such as model for end-stage liver disease (MELD-Na) score for prioritization of patients on waitlist for liver transplant (LT) are based. Due to impaired liver function, muscle wasting, decreased creatinine synthesis and increased tubular secretion at advanced stages of cirrhosis, baseline creatinine production is lower in patients with cirrhosis in comparison with that of the non-cirrhotic population. Furthermore, the sCr value is confounded by factors such as body weight, race, age, sex, total body volume, drugs, muscle metabolism, protein intake and interference in its laboratory assay by elevated bilirubin levels [6]. Another drawback in sCr is its inability to distinguish between AKI sub-types.
These lacunae in the currently available biochemical armamentarium for diagnosis and prognosis of AKI coupled with rising evidence of a ‘structural’ component of renal injury in HRS, have fuelled enthusiastic research in the field of novel biomarkers of AKI in DCLD [4, 5]. These biomarkers are small molecules (e.g. proteins or enzymes) that are released into the systemic circulation or urine as a result of changes in glomerular filtration rate, tubular cell injury or inflammatory cell infiltration [7]. Urine neutrophil gelatinase-associated lipocalin (NGAL) is one such promising tubular biomarker of AKI in DCLD, which has been the subject of extensive research in recent years [8,9,10,11,12]. While recent studies have primarily focused on exploring its role in the early identification of AKI and distinction between AKI sub-types, there is a dearth of literature analyzing its prognostic role in DCLD patients with or without AKI.
Methods
Study setting, duration and design
This was a single-center, prospective, analytical study conducted over a period of two years between June 2018 and June 2020 among 147 consecutive patients with DCLD, hospitalized at the SRM Institutes for Medical Science (SIMS), a tertiary care hospital in Chennai, southern India. The diagnosis of chronic liver disease (CLD) was based on a composite of clinical, biochemical, ultrasonographic, endoscopic and/or histological findings, with decompensation defined as the occurrence of jaundice, variceal bleed, ascites (uncomplicated, refractory or spontaneous bacterial peritonitis [SBP]), hepatic encephalopathy, renal dysfunction, non-SBP infections and hepatocellular carcinoma (HCC). Patients with advanced chronic kidney disease (CKD) already on renal replacement therapy (RRT) in the form of peritoneal dialysis or hemodialysis and those with prior renal and/or liver transplant (LT), were excluded.
Data collection
Baseline demographic, clinical and routine laboratory data such as complete blood counts, renal function tests, liver biochemistry and prothrombin time/international normalized ratio (INR) were recorded at the time of enrolment of patients into the study. Baseline sCr was defined as the nearest value estimated within three months prior to index hospitalization. When no prior sCr value was available, the sCr level at admission was considered as baseline. Urine samples collected on index admission were additionally sent for estimation of fractionated excretion of sodium (urine FENa) and protein creatinine ratio (urine protein/creatinine ratio [PCR]) along with urine NGAL.
Urine NGAL estimation
Quantitative urine NGAL measurement was performed using a commercially available enzyme-linked immunosorbent assay (NGAL ELISA Kit, Cat. E1719Hu Bioassay Technology Laboratory, Birmingham, UK). Estimations were carried out in duplicate following the manufacturer’s instructions and values were expressed as nanograms per milliliter [12].
Outcome assessment
Patients were assessed during index hospitalization for outcomes such as need for intensive care unit (ICU) admission, duration of ICU stay, length of hospital stay (LOHS), presence, sub-type and stage of AKI and death during index hospitalization. Patients who survived the period of index hospitalization were followed up telephonically or as outpatients as feasible until the end of the study period or up to mortality, whichever came earlier, and assessed for outcomes such as new-onset AKI (in patients who did not have AKI on index hospitalization), recurrence of AKI (in patients who had AKI on index hospitalization), number of recurrent hospitalizations and death during the follow-up. Primary outcomes were defined as all-cause mortality and time to death after index hospitalization. All other outcome measures were treated as secondary outcomes.
Operational definitions
AKI—diagnosis, sub-types and staging
AKI in DCLD was defined as per the adapted Kidney Disease Improving Global Outcome/International Club of Ascites (KDIGO/ICA) criteria [13,14,15,16,17]. Briefly, these were (i) acute increase in sCr of ≥ 0.3 mg/dL within 48 hours or (ii) increase in sCr by ≥ 50%, known or presumed to have occurred within the past seven days from a stable baseline sCr within three months. AKI was categorized into four stages as per modified ICA criteria [13, 15, 17]:
-
Stage I—increase in sCr ≥ 0.3 mg/dL or ≥ 1.5 – twofold from baseline (stage IA—sCr < 1.5 mg/dL, stage IB—sCr > 1.5 mg/dL)
-
Stage II—increase in sCr 2 – threefold from baseline
-
Stage III—increase in sCr > threefold from baseline or ≥ 4 mg/dL with an acute increase ≥ 0.3 mg/dL or initiation of RRT
AKI was further classified into three sub-types — pre-renal azotemia, HRS-AKI and intrinsic AKI or ATN—based on standard criteria and as per adjudication by treating team comprising three consultant gastroenterologists [13,14,15,16,17,18,19]. Pre-renal azotemia was diagnosed when there was a history of excessive fluid losses (i.e. excessive diuresis due to diuretic therapy with loss of body weight > 1 g/day or > 500 mg/day in patients with and without edema, respectively, or severe diarrhea) or bleeding (i.e. gastrointestinal bleeding as defined by hematemesis and/or melena) within few days before onset of AKI. HRS-AKI was diagnosed according to ICA criteria consisting of (a) presence of cirrhosis and ascites, (b) no improvement in sCr after two consecutive days of diuretic withdrawal and plasma volume expansion with albumin (1 g/kg body weight up to a maximum of 100 g per day), (c) absence of shock, (d) exclusion of recent/recurrent use of nephrotoxic agents and (e) exclusion of parenchymal kidney disease (absence of proteinuria (> 500 mg/day), absence of microhematuria (> 50 red blood cells per high-power field) and normal renal ultrasound [13, 14, 17,18,19]. Intrinsic AKI or ATN was diagnosed when three of the four following criteria were met: (a) FENa > 2%, (b) urinary osmolality < 400 mOsm/L, (c) urinary sodium > 40 mEq/L, and (d) presence of shock or use of nephrotoxic drugs.
Sample size estimation
Based on assumptions of a prevalence of AKI in DCLD of 40% as estimated from prior studies [1], a standard deviation of urine NGAL in the population of 100 ng/mL, and a difference of mean NGAL of 50 ng/mL between groups, with 80% study power and a type-I error of 5%, the required sample size was calculated as 133 patients with DCLD regardless of presence of AKI on index hospitalization.
Statistical analysis
Statistical analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) (IBM, SPSS Inc., Chicago, USA) software version 20. Statistical significance was set at a two-tailed p value of 0.05 for all analyzes. Biomarker values were expressed as median and interquartile ranges. Categorical variables were compared using Pearson’s Chi-square test. Kruskal–Wallis test was used to compare continuous variables. The area under receiver operating characteristic (AUROC) curves was used to assess the association between biomarkers and specific outcomes and to calculate optimum sensitive and specific cut-off values of urine NGAL for diagnosis of a specific outcome. Binary logistic regression analysis was performed to determine the association between urine NGAL and clinical outcomes (AKI and mortality related). The optimum cut-off value of urine NGAL ascertained from the ROC curves was used for plotting survival analysis using the Kaplan–Meier curve. Logarithmic adjusted urine NGAL levels were added to the corresponding MELD-Na score for plotting ROC curves to compare MELD-Na with composite MELD-Na-log urine NGAL score.
Ethics
The study protocol and consent forms were approved by the Institutional Ethics Committee of the SRM Institutes for Medical Science vide approval letter SIMS IEC/Other/04/2018.
Results
Baseline characteristics
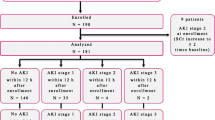
Of 147 patients hospitalized for various complications of DCLD during the study period, 139 were enrolled for final analysis after excluding eight patients who did not fulfill the inclusion criteria (Fig. 1). However, the specific enzyme-linked immunoassay (ELISA) kits used for NGAL estimation became unavailable soon after the onset of SARS-CoV-2 pandemic, and hence, urine NGAL estimation was available only in the first 100 patients enrolled in the study. The mean age of the study cohort was 56.9 years and 79.1% were male. Underlying etiologies for CLD were ethanol (39.6%), cryptogenic cirrhosis (26.6%), non-alcoholic fatty liver disease (NAFLD) (13.7%), hepatitis C virus (HCV) (9.4%), hepatitis B virus (HBV) (8.6%), drug-induced liver injury (DILI) (1.4%) and autoimmune hepatitis (0.7%), respectively. As many as 56 patients (40.3%) had some form of AKI at index hospitalization. Among these, 30.3% were classified as PRA, 46.4% as HRS-AKI and 23.2% as ATN. The median values for urine NGAL, urine protein:creatinine ratio (UPCR) and urine FENa were 208.1 ng/mL, 0.28 μg/g and 0.74%, respectively. The median MELD-Na score on admission was 21.3. 15.8% of patients belonged to Child–Pugh class A, 37.4% to class B and 46.8% to class C, respectively. The indications for hospitalization of the study cohort are listed in Table 1. A majority had an overlap of complications (both infectious and non-infectious etiology) at presentation. The mean duration of hospital stay was 5.5 days. As many as 30 patients required ICU stay during hospitalization and the median duration of ICU stay was 4.3 days. Twelve patients (8.6%) died during the index hospitalization. The remaining 127 patients were followed until the end of the study period or mortality, whichever came earlier. The baseline characteristics of patients stratified by the presence or absence of AKI during index hospitalization are shown in Table 2. Patients with AKI were more ill as compared to those without AKI with significantly higher mean Child–Pugh and MELD-Na scores, requirement of ICU care, in-hospital mortality, incidence of fluid overload, recurrent hospitalizations, overall mortality and a shorter mean time to death.
Discriminative value of urine NGAL
The median urine NGAL levels were highest in ATN (231.5 ng/mL), lowest in the PRA (163.3 ng/mL) and intermediate in HRS-AKI (212.4 ng/mL) (Table 3). Median sCr, urine FENa and UPCR also showed a similar trend. As shown in Fig. 2, urine NGAL was significantly different between PRA and HRS and between PRA and ATN, while both sCr and FENa were significantly different between PRA and ATN, but not between PRA and HRS. UPCR was unable to distinguish between AKI subtypes (p = 0.104). ROC curve analysis established that urine NGAL at a cut-off value of 203.9 ng/mL distinguished patients with HRS from those with PRA, with a sensitivity of 77.8% and specificity of 68.7% (AUC = 0.74, p = 0.016). On the contrary, urine NGAL did not significantly discriminate HRS-AKI from ATN (AUC 0.62, p = 0.328).
Box and whisker plots for assessment of discriminative value of urinary biomarkers. Center line is median, box shows interquartile range, whiskers show the maximum and minimum. PRA pre-renal azotemia, HRS hepatorenal syndrome, ATN acute tubular necrosis, uNGAL urine neutrophil gelatinase-associated lipocalin, FENa fractionated excretion of sodium, UPCR urine protein:creatinine ratio, MELD model for end-stage liver disease
Urine NGAL as a prognostic biomarker in index hospitalization
Twelve patients died during the index hospital admission. There was a significant correlation between baseline MELD-Na and urine NGAL values (p = 0.005) (Fig. 3). Urine NGAL levels significantly correlated with in-hospital mortality during the first admission (p = 0.04) and with need for ICU care (p = 0.014). However, it did not significantly correlate with length of hospital stay (p = 0.179) or length of ICU stay (p = 0.676).
Urine NGAL as a prognostic biomarker in the intermediate term
The mean duration of follow-up during the study period was 9.69 months. As many as 48.2% of patients, who had AKI during the index admission, had recurrence of AKI, while 25.3% of those who did not have AKI at index admission developed new-onset AKI during the follow-up period. Forty-five patients (32.4%) succumbed by the end of the study with a median time to death of 95 days. It was observed that an elevated baseline urine NGAL level (> 205 ng/mL) was associated with a significantly reduced overall survival at two years (p = 0.04) (Fig. 4). Comparison of baseline parameters according to urine NGAL levels is depicted in Table 4. Binary logistic regression analysis for evaluation of the association between urine NGAL levels and mortality at different time frames showed that elevated baseline urine NGAL levels were specifically associated with a higher three and six-month mortality regardless of the presence of AKI diagnosed by standard sCr-based criteria (p = 0.025 and p = 0.015, respectively) (Table 5). The AUC for urine NGAL for predicting three-month mortality was 0.69 (p = 0.012), with a best cut-off value of 209.95 ng/mL providing 73.7% sensitivity and 60.5% specificity. Similarly, AUC for predicting six-month mortality was 0.67 (p = 0.011), with a best cut-off value of 206.87 ng/mL providing 69.2% sensitivity and 60.5% specificity. However, elevated urine NGAL levels did not show significant correlation with other secondary outcomes such as recurrent hospitalizations (p = 0.445), new-onset AKI (OR 1.013, 95% CI 0.998–1.029) or recurrence of AKI (OR 1.011, 95% CI 0.999–1.023). Finally, we analyzed whether addition of urine NGAL to MELD-Na could improve the predictive performance of the MELD-Na score and concluded that a composite MELD-Na-log-urine NGAL score did not perform significantly better than the universally accepted MELD-Na score (ΔROC = 0.003) (Fig. 5).
Discussion
Urinary biomarkers for early diagnosis of AKI such as NGAL, interleukin-18, kidney injury molecule (KIM), liver type fatty acid binding protein (L-FABP) and FENa have been scrutinized across multiple studies published over the previous decade, albeit in the non-cirrhotic population [11]. A few studies have evaluated their role in early diagnosis/stratification of AKI into its sub-types in the ‘cirrhotic’ population and even fewer ones have explored the prognostic role of these urinary biomarkers [20,21,22,23,24,25,26,27,28]. Our study is one of the few prospective studies to have investigated both discriminant as well as prognostic function of urine NGAL in hospitalized DCLD patients.
Urine NGAL trends across various AKI sub-types in our study (i.e. highest in ATN, intermediate in HRS and lowest in PRA) were similar to those demonstrated in prior studies on a similar cohort by Verna et al., Ahmed et al. and Fagundes et al. [22,23,24]. In our patients, urine NGAL performed reasonably well (AUC = 0.74) in distinguishing HRS from PRA unlike other previously published studies; with an exception to a recent study by Udgirkar et al. [29]. The ability of urine NGAL to differentiate HRS from ATN seemed to be inferior to that of FENa. However, this could be because FENa is already included in the pre-specified definition for diagnosis of PRA (FENa < 1%) and ATN (FENa > 2%).
The traditional definition of HRS emphasizes an exclusively ‘functional’ nature of renal impairment [4, 5]. Recent studies have challenged this dogma and vouch for the contribution of sepsis and an additional ‘structural’ component to the pathogenesis of HRS [4, 5, 18, 19, 30,31,32,33]. Urine NGAL being a marker of tubular injury is thus expected to be elevated to a higher extent in HRS as compared to PRA, which, mechanistically, is a pure ‘functional’ form of renal impairment [22, 24]. This biological plausibility was further confirmed by our investigations.
Urine NGAL levels in patients with AKI were not significantly higher than in those without AKI. This was because a reduction in urine NGAL in PRA neutralized the rise in urine NGAL in the other two AKI groups. Further, studies have shown that urine NGAL is an early marker of kidney injury and that DCLD patients with elevated urine NGAL and normal sCr on admission have AKI-prone conditions and a higher likelihood of developing AKI in the course of time [26, 34, 35]. Another factor for the high urine NGAL in some DCLD patients without AKI could be the presence of bacterial infections in 40.4% of our patients. Barreto et al. have reported that urine NGAL levels were significantly elevated in DCLD patients with bacterial infections, regardless of presence or absence of AKI [21].
Our study results established an NGAL cut-off value (> 203.9 ng/mL) to distinguish HRS from PRA. A diagnosis of HRS mandates additional use of vasopressin analogues as opposed to volume expansion alone for management of PRA [5, 13, 17, 36]. Thus, routine use of urine NGAL may aid in earlier diagnosis of HRS, thereby allowing timely use of vasopressin analogues. In addition, it may also avoid wrongful wastage of resources in the management of PRA by restricting usage of vasopressin analogues to HRS-AKI. However, our results failed to provide an accurate cut-off value to distinguish HRS from ATN with good specificity and sensitivity. Currently, urine FENa and urine microscopy for casts are used to identify ATN in patients with DCLD [37, 38]. Further studies are needed to ascertain whether refining urine NGAL will help to accurately identify intrinsic AKI/ATN and distinguish it from HRS. This may help in refining patient management as the ATN sub-type responds neither to volume expansion with albumin nor to vasoconstrictor medication.
Urine NGAL levels generally correlated with markers of severity of liver disease such as MELD-Na score and Child–Pugh class and it assisted in short-term prognosis, as it correlated with need for ICU care and with in-hospital mortality. Previously, Belcher et al. have shown that baseline urine NGAL, along with a plethora of other urinary biomarkers viz IL-18, KIM-1, L-FABP and albuminuria, independently predicted AKI progression and in-hospital mortality after adjusting for confounding variables such as MELD score [20]. A study by Huelin et al. found urine NGAL values on day three of hospitalization and independently predicted 28-day mortality and progression of AKI [39]. Urine NGAL levels in the present study could neither predict recurrence of hospitalization nor development of new-onset/recurrent AKI during the follow-up period. Our study results established cut-off values for urine NGAL in predicting mortality at three and six months from the time of estimation. These findings are consonant with results of a previous study by Ariza et al., which concluded that lower levels of urine NGAL and urine albumin significantly predicted survival at three months regardless of presence or absence of acute-on-chronic liver failure (ACLF) [28]. Our study reported that urine NGAL < 205 ng/mL predicted survival at the end of the study period, i.e. 24 months, regardless of presence or absence of AKI on index hospitalization. The long-term prognostic impact of a single baseline value of urine NGAL has not been assessed prior to our study. Lastly, our investigations concluded that a composite MELD-Na-log-urine NGAL score did not significantly perform better than the MELD-Na score (Δ ROC = 0.003), contrary to a report by Lu et al. (Δ ROC = 0.163) [40].
This study had several limitations. Due to logistic issues, urine NGAL could not be estimated in a proportion of patients enrolled in the study. This happened due to lack of availability of the specific analytical kit that was used to analyze the initial 100 samples. The index hospitalization event, when urine NGAL was estimated, did not necessarily correspond to first instance of decompensation of CLD requiring hospitalization for each of the study participants. Our study design authorized for enrollment of a diverse cohort of DCLD patients at different stages in the natural history of their disease. This could have been a potential confounding factor influencing urine NGAL levels and their correlation with survival. Despite recruitment of a significant number of patients with AKI, the number of patients across AKI subtypes differed significantly. We did not serially monitor NGAL levels in our patients, and the role of urine NGAL in the prediction of AKI progression or development of nosocomial AKI during index hospitalization was not elucidated.
In summary, our study investigated the role of a single-point estimation of urine NGAL during hospitalization for any infectious or/and non-infectious complication of DLCD, in the stratification of AKI into its sub-types and prediction of short-term as well as long-term, clinically relevant outcomes. Median urine NGAL levels were found to differ across AKI sub-types. However, an optimal cut-off value that could stratify AKI into its sub-types could only be ascertained for differentiating HRS from PRA (> 203.9 ng/mL). Urine NGAL performed well in predicting need for ICU stay and in-hospital mortality during index hospitalization and mortality at three and six months after discharge. Survival at the end of the study period (24 months) was significantly lower in patients with elevated urine NGAL levels (> 205 ng/mL). Urine NGAL did not significantly add to the prognostic value of the MELD-Na score. Future research with a clutch of biomarkers that includes NGAL may well further refine the diagnostic and management algorithm of AKI in DCLD.
References
Bucsics T, Krones E. Renal dysfunction in cirrhosis: acute kidney injury and the hepatorenal syndrome. Gastroenterol Rep (Oxf). 2017;5:127–37.
Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–9.
Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–77.
Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis: International Ascites Club. Hepatology. 1996;23:164–76.
Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8.
Mullane JF, Gliedman ML. Development of renal impairment in Laennec’s cirrhosis. Ann Surg. 1971;174:892–901.
Ostermann M, Philips BJ, Forni LG. Clinical review: Biomarkers of acute kidney injury: where are we now. Crit Care. 2012;16:233.
Virzì GM, Clementi A, de Cal M, Cruz DN, Ronco C. Genomics and biological activity of neutrophil gelatinase associated lipocalin in several clinical settings. Blood Purif. 2013;35:139–43.
Rau S, Habicht A, Kauke T, et al. Neutrophil gelatinase-associated lipocalin and end-stage renal disease: it is not all about the kidneys! Eur J Clin Invest. 2013;43:816–20.
Ronco C, Legrand M, Goldstein SL, et al. Neutrophil gelatinase-associated lipocalin: ready for routine clinical use? An international perspective. Blood Purif. 2014;37:271–85.
Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54:1012–24.
Kift RL, Messenger MP, Wind TC, et al. A comparison of the analytical performance of five commercially available assays for neutrophil gelatinase-associated lipocalin using urine. Ann Clin Biochem. 2013;50:236–44.
Angeli P, Ginès P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–7.
Angeli P, Garcia-Tsao G, Nadim MK, Parikh CR. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–22.
Wong F. Acute kidney injury in liver cirrhosis: new definition and application. Clin Mol Hepatol. 2016;22:415–22.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-84.
European Association for the Study of the Liver. EASL Clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60.
Nadim MK, Kellum JA, Davenport A, et al. Hepatorenal syndrome: the 8th international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2012;16:R23.
Salerno F, Cazzaniga M, Merli M, et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241–8.
Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–32.
Barreto R, Elia C, Sola E, Moreira R, Ariza X, Rodriguez E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol. 2014;61(1):35–42.
Fagundes C, Pépin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–73.
Verna EC, Brown RS, Farrand E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012:2362–70.
Ahmed QA, El Sayed FS, Emad H, Mohamed E, Ahmed B, Heba P. Urinary biomarkers of acute kidney injury in patients with liver cirrhosis. Med Arch. 2014;68:132–6.
Slack AJ, McPhail MJ, Ostermann M, et al. Predicting the development of acute kidney injury in liver cirrhosis–an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther. 2013;37:989–97.
Treeprasertsuk S, Wongkarnjana A, Jaruvongvanich V, et al. Urine neutrophil gelatinase-associated lipocalin: a diagnostic and prognostic marker for acute kidney injury (AKI) in hospitalized cirrhotic patients with AKI-prone conditions. BMC Gastroenterol. 2015;15:140.
Gungor G, Ataseven H, Demir A, et al. Neutrophil gelatinase-associated lipocalin in prediction of mortality in patients with hepatorenal syndrome: a prospective observational study. Liver Int. 2014;34:49–57.
Ariza X, Graupera I, Coll M, et al. Neutrophil gelatinase-associated lipocalin is a biomarker of acute-on-chronic liver failure and prognosis in cirrhosis. J Hepatol. 2016;65:57–65.
Udgirkar S, Rathi P, Sonthalia N, et al. Urinary neutrophil gelatinase-associated lipocalin determines short-term mortality and type of acute kidney injury in cirrhosis. JGH Open. 2020;4:970–7.
Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233–43.
Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–81.
Bucsics T, Schwabl P, Mandorfer M et al. The trigger matters - outcome of hepatorenal syndrome vs. specifically triggered acute kidney injury in cirrhotic patients with ascites. Liver Int. 2016;36:1649–56.
Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382–91.
Aljumah AA, Tamim H, Saeed M, et al. The role of urinary neutrophil gelatinase-associated lipocalin in predicting acute kidney dysfunction in patients with liver cirrhosis. J Clin Med Res. 2018;10:419–28.
Jo SK, Yang J, Hwang SM, Lee MS, Park SH. Role of biomarkers as predictors of acute kidney injury and mortality in decompensated cirrhosis. Sci Rep. 2019;9:14508.
Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4:33.
Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3:1615–9.
Pepin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566–73.
Huelin P, Solà E, Elia C, et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology. 2019;70:319–33.
Lu J, Lin L, Ye C, et al. Serum NGAL is superior to cystatin C in predicting the prognosis of acute on chronic liver failure. Ann Hepatol. 2019;18:155–64.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Rohan Vijay Yewale, Balakrishnan Siddartha Ramakrishna and Giriprasad Venugopal. The first draft of the manuscript was written by Rohan Vijay Yewale and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
RVY, BSS, GV, BVD, and KR declare that they have no conflict of interest.
Ethics approval
The study was approved by the institutional ethics committee of SRM Institutes for Medical Science, Chennai, India (vide letter no SIMS IEC/Other/04/2018) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants for data collection and publication of this original article. A copy of the written consent is available for review by the Editor-in-Chief of this Journal.
Disclaimer
The authors are solely responsible for the data and the contents of the paper. In no way is the Honorary Editor-in-Chief, Editorial Board Members, the Indian Society of Gastroenterology or the printer/publishers responsible for the results/findings and content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yewale, R.V., Ramakrishna, B.S., Venugopal, G. et al. Urine neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury and prognosis in decompensated chronic liver disease: A prospective study. Indian J Gastroenterol 42, 106–117 (2023). https://doi.org/10.1007/s12664-022-01312-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12664-022-01312-w