Abstract
Purpose
Despite there being different materials for orbital floor reconstruction available today, outcomes are still not satisfying. In recent years, ultra-high molecular weight polyethylene (UHMWPE) has gained popularity in the field of orthopedic surgery due to its good biocompatibility and low infection rate. With its three-dimensional compound structure, it combines high stability and ductility, making it a potential material to be used for orbital floor reconstruction.
Methods
In a cadaver study, an overall of eighteen orbits were included. Fractures of the inferior wall were induced and then reconstructed using Polyglactin 910/PDS composite (Ethisorb) and UHMWPE (marPOR). Orbits were scanned by cone-beam CT in each condition: Intact, fractured and reconstructed with Ethisorb, marPOR 0.85 mm and marPOR 1.5 mm. Segmented orbital volumes were calculated by specialized software (Disior bonelogic CMF).
Results
All materials led to sufficient reconstruction of the initial orbital volumes (Ethisorb: p < 0.001; marPOR 0.85 mm: p = 0.003; marPOR 1.5 mm: p < 0.001). Orbits that were reconstructed with marPOR 0.85 mm showed the least mean volume difference from intact orbital volumes.
Conclusion
UHMWPE (marPOR) offers reliable reconstruction of orbital floor fractures combined with good stability, ductility and biocompatibility.
Similar content being viewed by others
Introduction
Orbital floor fractures are among the most frequent fractures of the midface [1]. Due to its delicate anatomical structures, the orbit is very susceptible to volume alterations caused by trauma. The most common blow-out-fracture enlarges the orbital volume, which in turn can lead to severe functional impairment, such as diplopia, enophthalmos, limitation of ocular movement and even vision loss [2]. Immediate surgical intervention to preserve the integrity of the orbit is therefore often necessary. In this context, restoration of preoperative orbital volume has proven to be the most useful numeric parameter for evaluating sufficient orbital reconstruction [3,4,5]. If there is no preoperative volume available, mirroring of the opposite orbit can also be used as reference [6]. Until today, polydioxanone (PDS) sheets are most commonly used for orbital floor reconstruction, followed by polyglactin 910/PDS composites and, for larger defects, titanium meshes [7]. However, the results are still not satisfying and around 20% of patients suffer from postoperative complications [8, 9]. Because of its high flexibility, PDS sheets and polyglactin 910/PDS composites gently adapt to the bony contour of the orbit, but on the other hand this leads to limited stability and higher rates of persisting enophthalmos and diplopia [7]. Therefore, both materials should be used only for small-to-moderate orbital floor fracture defects up to a maximum size of 1–2 cm2 (≙Jaquiéry I) [10]. Moreover, a significant number of patients with polyglactin 910/PDS implants experience postoperative infections [7, 8]. For larger defects, titanium mesh is widely used due to its stability and low distortion rate [11]. Despite titanium mesh, especially if pre-bent, offering reliable reconstruction of the orbital volume, fibrous reaction between the orbit and the mesh may prove devastating for the patient, causing cicatricial eye movement restriction and lid retraction [12, 13]. Beyond these serious complications, titanium implants can lead to tenderness, weather sensations and beam hardening in future x-ray examinations [14]. Therefore, the demand for a material that combines reliable orbital reconstruction, low infection rates and a flat, inert profile, rather than a mesh to prevent adhesions through the mesh, is unabated.
In recent years, ultra-high molecular weight polyethylene (UHMWPE) has become a popular material in the field of orthopedic surgery with excellent outcomes and low infection rates [15, 16]. It is radiolucent and cannot be imaged using x-rays. With its three-dimensional, highly porous compound structure, UHMWPE offers high stability combined with low dead weight. These properties make UHMWPE a suitable material to be used for orbital floor reconstruction. The aim of the present study is to investigate if UHMWPE is an equal material in terms of restoring preoperative orbital volumes in cadavers compared to a conventional polyglactin 910/PDS composite.
Material and Methods
The present study investigated an overall of eighteen orbits from nine human cadavers that were bequeathed to the Department of Anatomy, University of Erlangen-Nuremberg, Germany between 2018 and 2020. After cone-beam computed tomography (CBCT) images were taken from each cadaver head, inferior orbital walls were exposed via infraorbital approach and subperiosteal preparation. Thereafter, bilateral isolated trap-door fractures (≙Jaquiéry I) of the orbital floor were induced in a standardized manner using a raspatory (Fig. 1a–c), and all heads were rescanned. After reposition of herniated orbital tissue, UHMWPE implants (marPOR, KLS Martin Group, Tuttlingen, Germany) with a strength of 0.85 mm (Fig. 1d), 1.5 mm (Fig. 1e) and a Polyglactin 910/PDS composite sheet (Ethisorb, Ethicon, Johnson & Johnson, New Brunswick, NJ, USA) (Fig. 1f) were placed on the inferior orbital wall. All orbits were consecutively reconstructed with three different materials and rescanned in each case: marPOR 0.85 mm (Fig. 1d), marPOR 1.5 mm (Fig. 1e), Ethisorb (Fig. 1f).
Surgical procedure. View on the left eye from overhead (a). Infraorbital incision was performed (b) and fatty tissue was dissected. After incision of the periosteum on the inferior margo and subperiostal preparation into the orbit, the orbital floor was exposed and then fractured by using a sharp raspatory (c). Orbital floor defects were then reconstructed with different materials: marPOR with a strength of 0.85 mm (d), marPOR with a strength of 1.5 mm (e) and Ethisorb (f)
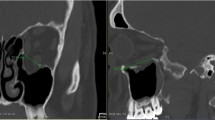
The intact, fractured (not reconstructed) and reconstructed orbital volumes (mL) were calculated automatically by using Disior Bonelogic CMF Orbital Software Version 2.1.30 (Disior, Helsinki, Finland). After importing images in digital imaging and communications in medicine (DICOM) format into the software, a segmented 3D model of the orbit was automatically created and turned into numerical data (Fig. 2a). Also, the fractured area (cm2) (Fig. 2b) and the maximum collapse (mm) were computed. Volume differences from intact orbital volumes were calculated for each case (not reconstructed, reconstructed with Ethisorb, marPOR 0.85 mm and marPOR 1.5 mm) and means were compared using the unpaired t-test (SPSS Version 28.0, SPSS Inc., Chicago, IL, USA). Data is expressed as box-and-whisker diagram, indicating minimum, maximum, median, first quartile and third quartile. A p-value lower than 0.05 was considered statistically significant.
Segmented 3D model of the orbital volumes and fracture area. Images in digital imaging and communications in medicine (DICOM) format were transferred into the CMF bonelogic orbital software (Disior, Helsinki, Finland). The program automatically generates a segmented 3D model of the orbit (a; indicated in white, contours in red) and turns into numerical data. Moreover, volumes of the fracture (a; indicated in purple), the fracture area (b; indicated in green) and the maximum prolapse into the maxillary sinus (not indicated) are automatically analyzed
Results
The mean absolute volume ± standard deviation (SD) of the intact orbits was 29.84 ± 3.27 mL (Fig. 3a, b). The mean volume ± SD of the fractured (not reconstructed) orbits was 30.68 ± 3.24 mL (Fig. 3c, d). All materials used for orbital reconstruction reduced absolute orbital volumes ± SD: 29.63 ± 3.63 mL (Ethisorb) (Fig. 4a, b), 29.79 ± 3.78 mL (marPOR 0.85 mm) (Fig. 4c, d), 29.5 ± 3.49 mL (marPOR 1.5 mm) (Fig. 4e, f).
Reconstructed orbits. Cone-beam CT scans of the right orbit in coronal (a, c, d) and sagittal (b, d, e) view. Each orbit was reconstructed using Ethisorb (a, b), marPOR with a strength of 0.85 mm (c, d) and marPOR with a strength of 1.5 mm (e, f). Segmented volumes (in red) were automatically created by specialized software
The mean absolute volume difference ± SD of the fractured (not reconstructed) orbit compared to the intact orbit (volume of fractured orbit – volume of intact orbit) was + 0.84 ± 0.71 mL. All investigated orbits exhibited higher volumes after the inferior wall was fractured (p < 0.001).
Compared to the fractured (not reconstructed) orbits, all reconstructed orbits showed significantly reduced absolute volume differences independent from the material that was used for orbital floor reconstruction (Ethisorb: p < 0.001; marPOR 0.85 mm: p = 0.003; marPOR 1.5 mm p < 0.001) (Fig. 5). Orbits that were reconstructed with marPOR 0.85 showed the least mean absolute volume difference ± SD (− 0.05 ± 0.95 mL) compared to Ethisorb (− 0.21 ± 0.93 mL) and marPOR 1.5 mm (− 0.34 ± 1.14 mL). All materials tended to overcompensate volume restoration, resulting in a smaller absolute mean volume compared to mean volume of intact orbits. However, these differences were not statistically significant.
Absolute volume differences from intact orbital volumes. Each box indicates minimum, maximum, median, first quartile and third quartile. All materials significantly reduced mean volume differences from the mean volume of fractured (not reconstructed) orbits: Ethisorb: p < 0.001; marPOR 0.85 mm: p = 0.003; marPOR 1.5 mm p < 0.001
The mean value ± SD of the fractured area was 1.54 ± 0.56 cm2. The mean maximal collapse ± SD was 3.74 ± 0.69 mm. All results are presented in Table 1.
Discussion
Although advances in biotechnology continue to introduce new materials for reconstruction of orbital floor defects, there is still inconclusive data about which material is best fit for orbital floor reconstruction [7]. However, current studies report PDS and Polyglactin 910/PDS composites, two materials which are widely used for orbital floor reconstruction, to be associated with increased infection rates and reduced ocular motility after surgery [7, 17, 18]. Due to their low stability, PDS and Polyglactin/PDS composites should be used only for defects smaller than 1–2 cm2 [10]. Titanium mesh offers higher strength and stability, but may cause tenderness, weather sensations, cicatricial eye movement disorders and beam hardening in x-ray examinations [12, 13, 19]. UHMWPE is an innovative biomaterial, which seemingly combines the advantages of PDS and titanium mesh, making it a suitable material for the reconstruction of orbital floor fractures. The present study is the first to investigate, if the use of UHMWPE leads to sufficient reconstruction of orbital volumes compared to a conventional Polyglactin 910/PDS composite patch (Ethisorb, Ethicon, Johnson & Johnson, New Brunswick, NJ, USA). For this purpose, UHMWPE (marPOR, KLS Martin Group, Tuttlingen, Germany) with two different strengths, 0.85 mm and 1.5 mm, was used. All materials led to statistically significant restoration of intact orbital volumes (orbital volumes before the fracture), but orbits that were reconstructed using marPOR with a strength of 0.85 mm exhibited the most accurate restoration with an absolute volume difference of − 0.05 mL from the mean volume of intact orbits. Using marPOR with a strength of 1.5 mm led to slight overcompensation. Mean orbital volumes tended to be smaller than intact orbital volumes with an absolute mean volume difference of − 0.34 mL, probably due to the simple size of the material. Even though Ethisorb is the thinnest material with a strength of 0.5 mm, orbits that were reconstructed using Ethisorb turned out to be 0.21 mL smaller on average compared to mean intact orbital volumes. Despite its thickness of 0.85 mm, marPOR offers a high ductility. The properties of UHMWPE are highly dependent on its microstructure rather than molecular mass, resulting in a very stable, but light-weight material with a molecular weight of 3.5–7.5 million g/mole [20, 21]. Furthermore, it provides a very high modulus of elasticity with 0.5–0.8 GPa. With its three-dimensional, highly porous compound structure, marPOR provides best conditions for neovascularization, osseointegration and therefore a good long-term stability [21]. Due to the porous structure, potential retrobulbar hemorrhage should not lead to any increase in retrobulbar pressure. The results and properties implicate that UHMWPE (marPOR) is not only very stable, preventing any soft tissue from prolapsing through the fracture, but also gently adapts to the bony contour of the orbit, which in turn leads to the most accurate volume restoration. In this context, it provides good manual ductility during the surgery. On the other hand, the stability of high-weight titanium mesh comes at the price of worse flexibility, demanding pre-contoured patient-specific titanium mesh for adequate orbital reconstruction, which is costly and time intensive, respectively [22]. In contrast to titanium mesh, UHMWPE is also radiolucent, does not affect any x-ray examinations and should not lead to tenderness or weather sensations [23]. Moreover, UHMWPE’s significance for achieving outstanding performances in total joint arthroplasties is unquestionably proven not only by its high wear-resistance, biocompatibility, durability, ductility and toughness, but also by the low infection rates below 1% of patients. [21, 24]. This may be a huge advantage over PDS as well as Polyglactin 910/PDS composites with reported infection rates up to 4% [7, 8]. However, further studies must clarify if this is also applicable in the context of orbital floor reconstruction. Because Polyglactin 910 and PDS are biodegradable materials, long-term stability, remnant defects and patient outcome significantly rely on the plates’ resorption rate. The risk of remnant defects have been increased as the plates had incomplete resorption, affecting one third of patients in a study of Tabrizi et al. [25]. This should not be seen in orbits reconstructed with marPOR as it is an inert, non-biodegradable material. We conclude that marPOR is an appropriate alternative material to be used for the reconstruction of orbital floor defects. However, clinical studies must be conducted in order to prove the effectiveness and safeness of marPOR in the context of orbital floor reconstruction. As it is already an approved CE medical product, marPOR could be used for orbital floor reconstruction today, minimizing any obstacles for further clinical evaluation.
References
Seifert LB, Mainka T, Herrera-Vizcaino C, Verboket R, Sader R (2021) Orbital floor fractures: epidemiology and outcomes of 1594 reconstructions. Eur J Trauma Emerg Surg. https://doi.org/10.1007/s00068-021-01716-x
Prabhu SS, Hemal K, Runyan CM (2021) Outcomes in orbital floor trauma: a comparison of isolated and zygomaticomaxillary-associated fractures. J Craniofac Surg 32(4):1487–1490. https://doi.org/10.1097/SCS.0000000000007418
Fan X, Li J, Zhu J, Li H, Zhang D (2003) Computer-assisted orbital volume measurement in the surgical correction of late enophthalmos caused by blowout fractures. Ophthalmic Plast Reconstr Surg 19(3):207–211. https://doi.org/10.1097/01.iop.0000062848.26273.e5
Ebrahimi A, Kalantar Motamedi MH, Rasouli HR, Naghdi N (2019) Enophthalmos and orbital volume changes in zygomaticomaxillary complex fractures: Is there a correlation between them? J Oral Maxillofac Surg. 77(1):134 e131-134 e139. https://doi.org/10.1016/j.joms.2018.08.028
Schonegg D, Wagner M, Schumann P et al (2018) Correlation between increased orbital volume and enophthalmos and diplopia in patients with fractures of the orbital floor or the medial orbital wall. J Craniomaxillofac Surg 46(9):1544–1549. https://doi.org/10.1016/j.jcms.2018.06.008
Jansen J, Dubois L, Schreurs R et al (2018) Should virtual mirroring be used in the preoperative planning of an orbital reconstruction? J Oral Maxillofac Surg 76(2):380–387. https://doi.org/10.1016/j.joms.2017.09.018
Avashia YJ, Sastry A, Fan KL, Mir HS, Thaller SR (2012) Materials used for reconstruction after orbital floor fracture. J Craniofac Surg 23(7 Suppl 1):1991–1997. https://doi.org/10.1097/SCS.0b013e31825aada1
Gosau M, Schoneich M, Draenert FG, Ettl T, Driemel O, Reichert TE (2011) Retrospective analysis of orbital floor fractures–complications, outcome, and review of literature. Clin Oral Investig 15(3):305–313. https://doi.org/10.1007/s00784-010-0385-y
Bourry M, Hardouin JB, Fauvel F, Corre P, Lebranchu P, Bertin H (2021) Clinical evaluation of the efficacy of materials used for primary reconstruction of orbital floor defects: meta-analysis. Head Neck 43(2):679–690. https://doi.org/10.1002/hed.26518
Buchel P, Rahal A, Seto I, Iizuka T (2005) Reconstruction of orbital floor fracture with polyglactin 910/polydioxanon patch (ethisorb): a retrospective study. J Oral Maxillofac Surg 63(5):646–650. https://doi.org/10.1016/j.joms.2004.11.013
Degala S, Shetty SK, Biddappa L (2013) Reconstruction of post-traumatic internal orbital wall defects with titanium mesh. J Maxillofac Oral Surg 12(4):418–423. https://doi.org/10.1007/s12663-012-0444-9
Kersey TL, Ng SG, Rosser P, Sloan B, Hart R (2013) Orbital adherence with titanium mesh floor implants: a review of 10 cases. Orbit 32(1):8–11. https://doi.org/10.3109/01676830.2012.736597
Lee HB, Nunery WR (2009) Orbital adherence syndrome secondary to titanium implant material. Ophthalmic Plast Reconstr Surg 25(1):33–36. https://doi.org/10.1097/IOP.0b013e3181929b6e
Verweij JP, Hassing GJ, Fiocco M, Houppermans PN, van Merkesteyn JP (2016) Removal of osteosynthesis material because of symptoms after Le Fort I osteotomy: a retrospective study of 158 patients. J Craniomaxillofac Surg 44(12):1909–1912. https://doi.org/10.1016/j.jcms.2016.09.009
Pietrzak WS (2021) Ultra-high molecular weight polyethylene for total hip acetabular liners: a brief review of current status. J Invest Surg 34(3):321–323. https://doi.org/10.1080/08941939.2019.1624898
Sobieraj M, Marwin S (2018) Ultra-high-molecular-weight polyethylene (UHMWPE) in total joint arthroplasty. Bull Hosp Jt Dis 76(1):38–46
Kos M, Brusco D, Engelke W (2006) Results of treatment of orbital fractures with polydioxanone sheet. Polim Med 36(4):31–36
Gierloff M, Seeck NG, Springer I, Becker S, Kandzia C, Wiltfang J (2012) Orbital floor reconstruction with resorbable polydioxanone implants. J Craniofac Surg 23(1):161–164. https://doi.org/10.1097/SCS.0b013e3182413edc
Shah HA, Shipchandler T, Vernon D et al (2018) Extra-ocular movement restriction and diplopia following orbital fracture repair. Am J Otolaryngol 39(1):34–36. https://doi.org/10.1016/j.amjoto.2017.08.008
Bracco P, Bellare A, Bistolfi A, Affatato S (2017) Ultra-high molecular weight polyethylene: influence of the chemical, physical and mechanical properties on the wear behavior: a review. Mater Basel 10(7):791. https://doi.org/10.3390/ma10070791
Hussain M, Naqvi RA, Abbas N et al (2020) Ultra-high-molecular-weight-polyethylene (UHMWPE) as a promising polymer material for biomedical applications: a concise review. Polymers Basel 12(2):323. https://doi.org/10.3390/polym12020323
Sigron GR, Ruedi N, Chammartin F et al (2020) Three-dimensional analysis of isolated orbital floor fractures pre- and post-reconstruction with standard titanium meshes and “hybrid” patient-specific implants. J Clin Med. 9(5):1579. https://doi.org/10.3390/jcm9051579
Kozakiewicz M, Elgalal M, Walkowiak B, Stefanczyk L (2013) Technical concept of patient-specific, ultrahigh molecular weight polyethylene orbital wall implant. J Craniomaxillofac Surg 41(4):282–290. https://doi.org/10.1016/j.jcms.2012.10.007
Gomez-Barrena E, Puertolas JA, Munuera L, Konttinen YT (2008) Update on UHMWPE research: from the bench to the bedside. Acta Orthop 79(6):832–840. https://doi.org/10.1080/17453670810016939
Tabrizi R, Langner NJ, Pouzesh A, Arabion H (2013) Evaluation of the biodegradable plates (PG910/PDO) for reconstruction of various sizes of orbital floor defects in the blow-out fractures. Craniomaxillofac Trauma Reconstr 6(3):187–190. https://doi.org/10.1055/s-0033-1349205
Acknowledgements
We thank Anna-Maria Henell and Claire Rivoire-Kunze from DISIOR for providing a free version of CMF bonelogic orbital and for their great support.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YF and RP. The first draft of the manuscript was written by YF and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Frank Reinauer and Adem Aksu are employees of KLS Martin Group, Tuttlingen, Germany. The remaining authors have no conflicts of interest to declare.
Consent to Publication
All donors had voluntarily donated their bodies to medical research.
Ethics Approval and Consent to Participate
This study did not require ethics approval.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Foerster, Y., Kesting, M., Reinauer, F. et al. Ultra-High Molecular Weight Polyethylene (marPOR) is a Suitable Material for the Reconstruction of Orbital Floor Fracture Defects in Human Cadavers. J. Maxillofac. Oral Surg. (2022). https://doi.org/10.1007/s12663-022-01789-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12663-022-01789-0