Abstract
The slaughterhouse and meat industry are worldwide rapidly growing and produce billions of tonnes of organic wastes annually. These materials can be used to produce biogas through anaerobic digestion and the resulting digestate represents a potential source of organic carbon and nutrients that could be applied to the soil as organic fertilizer. The present work aims to assess the fertilizing potential of a dried anaerobic digestate (DD) produced from beef slaughtering waste. DD was characterized at the physic-chemical level, particularly focusing on macro- and micronutrient contents, potentially toxic element and organic contaminants. Then, a short-term soil incubation experiment was performed on two different soils. After the incubation, DD released 10–26% of their total nitrogen (N) and 13–16% of total phosphorus (P), depending on the soils that had different characteristics and responded differently to the treatments. However, DD had positive effects on the principal soil fertility indicators, such as chitinase and phosphatases, stimulating the microbial activity and therefore exploiting a fertilizing potential as well as the other organic fertilizers tested. Moreover, DD had minor effects on soil extractable carbon (Cext) suggesting the presence of recalcitrant C forms in spite of soluble C, indicating a higher stability of slaughterhouse by-products after anaerobic digestion in respect to the other organic fertilizers. The results obtained in this work raise the concrete possibility use DD as a bio-based fertilizer.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Statement of novelty
A dried anaerobic digestate from slaughterhouse wastes was characterized and tested for their fertilizing potential in a short-term soil incubation experiment. The digestate was able to provide nutrients to the soil, stimulating the microbial community and having a positive impact on the principal indexes of the soil fertility. These results pose concrete perspective for the use of slaughterhouse by-product as a bio-based fertilizer after anaerobic digestion, opening new horizons regarding the use of slaughterhouse residues as fertilizer in a circular economy-model.
Introduction
The use of alternative energy from natural sources and the improvement of waste management are key steps to face the current global challenges, exacerbated by the “Great Acceleration” of anthropogenic activity [1]. In this scenario shifting from a linear economy model toward circular economy would create sustainable business by minimizing the resource consumption allowing the recycle and reuse of products and materials [2, 3]. In agriculture, streams of waste materials can be channelled through biogas plant, in which fermentative processes produce biogas and digestate, which is a by-product of fermentative process occurring during anaerobic digestion [4]. In Europe, around 180 million tonnes of digestate are produced annually, of which 120 million tonnes come from agricultural biogas plants [5]. In the European countries, the major sources for biogas production are corn silage, cattle slurry and manure, but potentially every organic waste and by-products from the agrifood industry are suitable for anaerobic digestion [6]. The digestate can be converted into organic fertilizers and then applied to the fields, thereby improving the energy-recovery rate of waste materials through agriculture [7], Reg. (EU) 2019/1009).
The slaughterhouse industry generates billions of tons of organic waste annually, presenting a substantial potential reservoir for utilization in anaerobic digestion processes aimed at energy production [8, 9]. The slaughterhouse residues consist of a solid part which comprise stomach, bowels and inedible parts, and wastewater that can contain blood, oil, fats, salts, suspended solids [10]. The solid phase of the slaughterhouse wastes can be categorized into two types: the first consisting of ruminal, stomach, intestinal contents and dung, the second consisting of inedible offals, tissues, and meat trimmings [8, 11]. These materials exhibit elevated levels of organic matter, protein, and lipid constituents, which hold promise for serving as valuable nutrient sources for agricultural crops [12]. In this view, anaerobic digestion is recognized as a viable pretreatment approach sanctioned by Regulation (EC) 1069/2009 for the management of animal by-products before to be disposed to the field.
Soil fertility is defined as the ability of soil to support plant growth by providing the essential nutrients and adequate chemical, physical, and biological conditions and to sustain the maintenance of ecosystem services [13]. The potential of organic wastes to improve soil fertility in an healthy soil is a key requisite of circular economy models [14]. Several studies showed that different types of anaerobic digestate (AD) from crop residues [15], energy crops [16], municipal wastes [17], livestock slurry [18] and cattle manure [19] can be used in agriculture as partial replacement of mineral fertilizers. These different digestates have been related as potential carrier of organic matter and nutrients for crops growth, with a positive impact on soil fertility [15, 20, 21]. Particularly, AD from cattle slurry can increase soil total organic carbon (TOC), nitrogen (N), phosphorus (P) and potassium (K) contents [22,23,24]. However, researches on AD from slaughterhouse residues used as fertilizer are few, and were mostly studied in mixture with other materials [25,26,27,28,29]
In order to direct the AD toward an agricultural use it is essential to evaluate the principal soil fertility indicators, which regard the capability of providing organic C and nutrients availability and the metabolic activity of the microbial biomass [30, 31]. However, as other organic and inorganic fertilizers, ADs could have some undesirable effect on the environment if spread without a correct management [18]. Therefore, a relevant characterization of ADs composition is essential to assure sustainability and reliability prior their application in the soil [20, 31].
The present work aims to assess the fertilizing potential of a dried anaerobic digestate (DD) produced from beef slaughterhouse wastes. The approach of this work followed a physico-chemical characterization of DD, particularly focusing on macro- and micronutrient contents, potentially toxic element and organic contaminants. Afterwards, two agricultural soils with distinctive characteristics were fertilized in pots with DD, two organic fertilizers (cattle manure, CM and digested manure, DM) and a mineral fertilizer (MIN) to compare their fertilizing potential. The release of N and P were monitored during the incubation period. Then, at the end of the incubation, analyses regarding the impact on soil biochemical indicators and community level physiological profiling (CLPP) were performed. Furthermore, soil chemical parameters were investigated. Considering that, by now, there is not an EU regulation for the use of ADs from animal by-products, the results obtained in this work could be important in the preparation of a guideline for the DD use in agriculture.
Materials and Methods
Dried Anaerobic Digestate Production
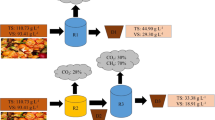
The dried anaerobic digestate (DD) from slaughterhouse by-products was produced in June 2021 by INALCA S.p.A. (https://www.inalca.it/en/) in their own biogas plant located in Pegognaga (Italy). The digestate produced derived from the anaerobic co-digestion of (i) sludge from the INALCA wastewater plants; (ii) stomach content (slaughter by-product); (iii) blood. The row incoming materials were pasteurised, shredded and mixed and finally subjected to mesophilic anaerobic wet digestion, which lasted for 35 days. Liquid–solid separation was operated by centrifugation, followed by a drying treatment performed through a belt dryer that allowed the digestate to reach a humidity content around 20%. The process is summarized in Fig. 1. The final DD sample was composed of five subsamples of 4 kg each, taken with a clean shovel at two-hour intervals during the production activity. According to the INALCA’s production rates, the resulting 20 kg sample could represent a production of about 10 tons of dried digestate. The sample was then homogenised, milled and sieved at 0.5 mm before the analyses.
Dried Anaerobic Digestate Characterization
The main physicochemical characteristics of DD and the other product tested are listed in Table 1 and were determined according to the European methods for fertilizers (https://eur-lex.europa.eu/eli/reg/2003/2003/oj). The chemical reaction (pH) was measured in 3:50 mass-to-water ratio extract after 30 min of shaking at room temperature (RT). The electrical conductivity (EC) was measured in the 1:10 mass-to-water ratio after 30 min of shaking at RT. Total- (TS) and volatile solids (VS) and ashes were determined, reaching a constant weight at 105 and 450 °C. Total organic carbon (TOC) was determined by wet oxidation with potassium dichromate method [32]. Total nitrogen (TN) was determined after acid digestion with sulfuric acid and selenium-potassium persulfate as catalyser, using a Kjeldahl automatic instrument (KjelFlex K360, BUCHI Labortechnik AG, Flawil, Switzerland). The ammonium and nitrate N were determined after extraction with 1 M KCl (1:10 w:v) and steam distillation with Kjeldahl automatic instrument after adding magnesium oxide for ammonium N and Devarda alloy for nitrate N [33]. The total organic nitrogen (TON) was calculated by the difference between total N and inorganic N. Total phosphorus (P), total sulphur (S) and metals were determined by microwave wet acid digestion (Start-E, Milestone, U.S.A.) and by inductively coupled plasma optical emission spectrometer (Spectro Arcos ICP-OES Analyzer, Spectro Analytical Instrument GmbH, Kleve, Germany). Hexavalent chromium (CrVI) was determined in agreement with the protocol ISO 17075-2:2017. The oxygen uptake rate (OUR) was determined following the protocol ISO 16087-1:2020. Enumeration of faecal coliforms (Escherichia coli) and Salmonella spp. was obtained with the procedure ISO 7251:2005 and US EPA 1682 [34]. Finally, the mono- and polycyclic aromatic hydrocarbons (PAH) were determined with the protocols EPA 5021A 2014 + EPA 8260D 2018 and EPA 3550C 2007 + EPA 8270E 2018 respectively. The DD was analysed for the P fractions after subsequential extractions in deionized water (fraction 1), NaHCO3 0.5 M pH 8.5 (fraction 2), NaOH 0.1 M (fraction 3), and HCl 1 M (fraction 4) basing on [35]. Briefly, 0.30 g of DD was added to 30 mL of the first reagent (water) and shacked for 24 h, then it was centrifuged with Beckman Coulter centrifuge and JA-10 rotor at 26,550 RCF for 30 min and filtered. Pellet was re-solubilized with the same amount of the next reagent. The procedure was repeated until the last reagent (HCl) and supernatants were analysed for P through ICP-OES instrument.
Soil Incubation Experiment
Soils Description
The soil incubation experiment was carried out onto two agricultural soils located in Northern Italy with different physico-chemical characteristics and fertility. The first sample (FC) was collected from the topsoil (0–0.2 m) of a Fulvic Cambisol soil (WRB, 2014) from the University of Bologna experimental farm, located in Cadriano in the southern part of Po Valley, Italy (45.53° N, 11.38° E, 28 m a.s.l., annual average temperature 14.7 °C, annual average precipitation 621 mm); The second sample (CC) was collected in Quinto Vicentino (VI) from the topsoil (0–0.2 m) of a Clay-loam Cambisol soil (WRB, 2014) (45.15° N, 11.15° E, 35 m a.s.l., annual average temperature 13.3 °C, annual average precipitation 1103 mm).
Experimental Design
Soil subsamples were air dried, milled, sieved at 2 mm and analysed in agreement with SSSA methods [36], and the results are reported in Table S1. The soil samples were preincubated at 20 ± 2 °C and 30% of full water holding capacity for 14 days. The DD, digested manure-based fertilizer (DM), cattle manure organic fertilizer (CM) and a positive control constituted by diammonium phosphate (MIN) were added to the soil at 100 mg N kg−1, corresponding approximately to 240 kg ha−1 of N. Treatments were compared to untreated soils used as control (CTR). Each treatment was carried out in triplicate and the pots were incubated at 20 ± 2 °C in the dark for 6 weeks (42 days). Moisture was kept approximately constant during the incubation by weighing the pot weekly and adding deionized water when necessary. After 0, 3, 7, 14, 21, 28, 35, 42 days the pots were sampled and analysed for extractable inorganic ammonium–N and nitrite-nitrate–N and available (Olsen) P. The 3 days- time sampling was excluded for available P. The pots were then sampled at the end of the incubation for biochemical indicators and community level physiological profile (CLPP). During these analyses, soils were kept in a cold room at 4 °C for one week. Then, the soil samples were air dried and analysed for chemical indicators.
Inorganic N and Available P Release
Ammonium-N (NH4+-N) and nitrite/nitrate–N (NO3−-N + NO2−-N) were extracted with a 1 M KCl (1:10, w/v) for 2 h at RT and determined colorimetrically using a flow autoanalyzer (AA3, Bran Lubbe, Germany). Available P was extracted with 0.5 M NaHCO3 pH 8.5 [37] and determined ICP-OES.
Biochemical Indicators
Soil extractable organic C and N (Cext and Next) were extracted by 0.5 M K2S04 in a ratio of 1:4 with fresh soil for 30 min and determined by OC-VCPH/CPN (Shimadzu, Japan). Soil microbial biomass C was determined through the fumigation-extraction method [38].
Alkaline and acid phosphomonoesterase activities were determined following the method of Eivazi and Tabatabai [39]. β-glucosidase activity was determined as described by Eivazi and Tabatabai [40]. N-acetyl-β-D-glucosaminidase (NAG) activity was determined according to Parham and Deng [41]. These enzymatic activities were expressed as mmol of PNP kgdw−1 h−1. Urease activity was determined according to Kandeler and Gerber [42] and expressed as mmol N kgdw−1 h−1. Tyrosinase activity was measured as described by Sinsabaugh et al. [43] and expressed as mmol DOPA kgdw−1 h−1. Dehydrogenase activity was determined according to von Mersi and Schinner [44] and the activity was expressed as mmol INTF kgdw−1 h−1.
Community Level Physiological Profiling (CLPP)
Functional analysis of soil microbial community (CLPP) was performed following the method described by using 96-well EcoPlates™ containing 31 carbon-based substrates and a water control colorimetrically read by Biolog® device (OmniLog, U.S.A.). Fresh soil samples corresponding to 2 g of dry soil were extracted in 20 mL NaCl 0.85% (w/v), kept for 2 h in agitation and decanted for 1 h. The supernatants were diluted 3.16:10 with extracting solution. EcoPlates™ were incubated with 100 µL per well, each plate containing three replicates of every soil treatment, for a total of 5 plates incubated with FC samples and 5 with CC samples. OmniLog device was set at 25 °C and plates were incubated for 7 days, measuring absorbance at 590 nm every 30 min. Data were analysed selecting measures taken every 4 h. A total time of 120 h of incubation was reported as the optimal range of optical density readings giving the most reliable data [45], thus 120 h of incubation results were chosen as endpoints.
Chemical Indicators
Soil pH and electrical conductivity (EC) were determined in agreement with SSSA methods [36]. Exchangeable magnesium (Mg) and K were extracted from 1 g of soil in 20 mL of hexammine cobalt chloride 0.017 M saturated with CaCO3 according to ISO 23470:2018 method and determined by ICP-OES.
Statistical Analyses and Data Handling
The statistical analysis of soils incubation experiments followed a completely randomised experimental design and were performed in the R environment using Rstudio version 2021.09.2 Build 382 [46]. Data were analysed through one-way ANOVA. To verify the ANOVA assumptions, Shapiro–Wilk’s test was used to assess the normality of distributions and Levene’s test for homogeneity of variances. The significance of tests was assessed at p-value less than or equal to 0.05. Post hoc HSD Tukey’s test was performed to investigate differences between treatments when ANOVA returned a significant global test. Data obtained from CLPP were analysed as average well colour development (AWCD), which describes the average use of C-sources by the microbial community. The AWCD was calculated according to Ge et al. [47] as the sum of the difference between the optical density at 590 nm (OD590) of each C-source-containing well and the control well:
where Sw is the OD of a substrate well, Cw is the OD of the control (water) well, n is the number of substrates (n = 31). AWCD was calculated every 4 h from 0 to 120 h.
Principal Component Analysis (PCA) was performed as a multivariate analysis of Cext and Next, enzyme activity and Microbial Biomass Carbon (MBC) using the “prcomp” function in the R environment [46], then two-way ANOVA was carried out on PCA scores considering treatment and soil factors.
Results
Digestate Characterization
Table 1 shows the main characteristics of DD beside the characteristics of the other products used in the soil incubation experiment. As expected for dried materials, total solid ranged between 88.5 (in DD) and 92.1% fw for all the products tested. Volatile solid content was the highest in DD (81.4% dw) and never lower than 71%. The pH was neutral (7.3 in DD) with the exception of digested manure (DM) in which was 9.75 and the electrical conductivity was the lowest between the other products (2.48 dS m−1) which however does not exceed 9.4 dS m−1. Total organic C (TOC) ranged between 33.9 and 43.3% dw (40.3 in DD) whereas N was the highest in DD (5.0% dw). C/N ratio resulted 8.0 in DD and over 10 for the other products. The TN content was the highest (4.4% dw more than 85% of total N) as well as the total P content (2.4% dw). In DD, the most abundant P-fraction was the H2O-P which accounted for 28.1%, followed by the HCl-P fraction, which accounted for 27.1%; the NaOH-P fraction accounted for 2.98%, whereas 1.51% was represented by NaHCO3-P; total recovered P, as the sum of each fraction, accounted for 60% whereas the unrecovered part can be considered insoluble residual P. Excluding calcium (3.2% dw) other nutrients were present in concentration equal (total S) or lower than 1% dw. The concentrations of potentially toxic elements (i.e., As, Cd, CrVI, Cu, Hg, Ni, Pb, etc., Table 1), mono- and polycyclic aromatic hydrocarbons (data not shown), and microbiological indicators (i.e., oxygen uptake rate, Escherichia coli and Salmonella spp., Table 1) were lower than the limits imposed by European legislation for anaerobic digestates (Reg. (EU) 2019/1009) and by the Italian regulation for similar organic fertilizers (D.Lgs. 75/2010).
Soil Incubation
Inorganic N and Available P Release
Table 2 reports the results of ammonium-N and nitrite/nitrate–N released in the two soils treated with DD, mineral fertilizer (MIN), digested manure (DM) and undigested cattle manure (CM) during the incubation period of 42 days. Nitrogen release kinetics for all the treatments were slower in CC than FC soil: ammonium-N was released within 7 in FC whereas it was released within 21 days in CC, then ammonium-N release became negligible (Table 2). After 3 days of incubation in FC and 14 days in CC, inorganic N was found mostly as nitrite/nitrate–N and the highest concentration was observed in MIN treatment (Table 2). At the end of the incubation, MIN reported highest values for nitrite/nitrate–N followed by DD in FC soil, whereas in CC soil MIN reported the highest values and no differences for the other treatments with untreated soils (CTR). DM did not show any increasing trend in inorganic N during the incubation (Table 2).
Table 3 reports the available (Olsen) P release trend in FC and CC soils. All the treatments showed available P values that remained constant during the incubation in both soils. The highest available P was observed after the treatment with MIN (Table 3) either in FC or in CC soil (Table 3). DM and DD reported the second highest values and showed no statistical differences in FC soil, whereas in CC soil DM released more P than DD at 14, 21, 35 and 42 days. CM always reported the lowest values in both soils.
Biochemical Indicators
Extractable organic C (Fig. 2A) in FC soil reported the only significant difference between DD and CM (p = 0.025), whereas in CC was significantly increased by all the treatments, with highest values reported by CM, in respect to CTR (p < 0.001). Extractable N values were about 1.5 times higher in CC soil than FC (Fig. 2B) and reported the highest values in MIN treated soils (p < 0.001). However, DD and CM treatment increased Next while DM did not increase Next in CC soil and decreased it in FC (p < 0.001). Microbial biomass carbon (MBC) was significantly increased by DD treatment in FC (p < 0.001) with highest values in DM and CM, but only DM increased MBC in respect to untreated soils in CC (Fig. 2C).
A Extractable C, B extractable N and C microbial biomass carbon (MBC) grouped by soil (FC, CC) after 42 days of incubation with the different treatments: control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM). Data shown are the mean of three replicates; error bars indicate one standard errors of the mean; dw = dry weight; different letters indicate a significant difference for p < 0.05 and are based on one-way ANOVA with p < 0.05 HSD Tukey’s test
Results of soil enzymatic activities are reported in Fig. 3. In both soils, the trend is generally a higher increase in soil enzymatic activities consequent to the treatment with CM, followed by DM and DD in respect to CTR (p < 0.001), except for chitinase (NAG) activity in FC in which DM reported higher values (p < 0.001) and urease in CC that was decreased by CM and MIN and not affected by the other treatments (p < 0.001). Tyrosinase activity was decreased by the treatments in FC (p = 0.01) and significantly increased in CC only by CM (p = 0.004) in respect to untreated soils. In general, MIN decreased or not affected enzymatic activities (Fig. 3).
Acid phosphatase A, alkaline phosphatase B, β-glucosidase C, chitinase D, tyrosinase E, urease F and dehydrogenase G activities grouped by soil (FC, CC) after 42 days of incubation with the different treatments: control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM). Data shown are the mean of three replicates; error bars indicate one standard errors of the mean; different letters indicate a significant difference and are based on one-way ANOVA with p < 0.05 HSD Tukey’s test
Community Level Physiological Analysis (CLPP)
Average well colour development (AWCD) was calculated as an index of substrate degradation given by the sum of the average metabolic response (AMR) divided by the number of substrates (n = 31) [48]. Figure 4A shows that AWCD after 120 h of incubation in Biolog™ system evidenced no significant differences in FC soil among treatments (p = 0.65). Conversely, in CC soil DD, DM and CM increased AWCD values that were significantly higher by MIN, whereas only DD and DM reported statistically higher values than CTR (p < 0.001). The relative amount of the different groups of substrates (amino acids, amines and amides, carbohydrates, carboxylic and keto acids and polymers) were reported in relative percentage in Fig. 4B for FC and CC soil respectively. Generally, the image indicates that no great changes in the degradation of the considered group of substrates occurred after the treatments. However, in both soils amines and amides (Am_Am) were not degraded by the microbial community after MIN treatment. Moreover, in CC soil CM also did not report the degradation of Am_Am. In general, in CC soil this group of substrates was less represented in all the treatments. The relative degradation of polymers (Poly) reported a higher variation in FC soil depending on the treatment in spite than in CC soil, but it was always present in all the treatments, as well as the other group of substrates.
Results of the CLPP analyses with EcoPlates™ on FC and CC soils performed after 42 days of incubation: A AWCD values after 120 h of incubation for CLPP analyses; B relative percentage of substrate consumption per group of substrates (AA: amino acids; Am_Am: amines and amides; Carb: carbohydrates; Carb_Ket: carboxylic and keto-acids; Poly: polymers). Different letters represent different treatments and are means of AWCD calculated every 4 h. Control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM)
Chemical Indicators
Soil pH at the end of the incubation period ranged between 7.67 and 7.90 (Fig. 5A), and all treatments decreased it significantly in both soils (p < 0.001 in FC; p = 0.0014 in CC). The fertilizer that decreased pH the most was MIN, followed by CM, digested manure DM and DD. Electric conductivity (EC, Fig. 5B) of soils after the incubation with the different fertilizers ranged between 0.23 dS m−1 in CTR in FC soil and 0.42 dS m−1 in MIN in CC soil. Generally, CC soil reported higher values than FC and MIN was the treatment that increased EC the most, followed by CM, DD and DM in respect to untreated soils (p < 0.001).
A pH and B electric conductivity (EC) grouped by soil (FC, CC) after 42 days of incubation with the different treatments: control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM). Data shown are the mean of three replicates; error bars indicate one standard errors of the mean; DW = dry weight; different letters indicate a significant difference for p < 0.05 and are based on one-way ANOVA with p < 0.05 HSD Tukey’s test
Figure 6 reports the results of analysis of exchangeable Mg, K and Ca in soils at the end of incubation. Exchangeable Mg reported the higher values in DM and CM in FC soil (p < 0.001), whereas in CC soil Mg was mostly increased by MIN, followed by DM and DD (p < 0.001, Fig. 6A). Exchangeable K showed overall higher values in FC than CC soil, but in both soils was increased only by DM and CM treatments (p < 0.001, Fig. 2B). Exchangeable Ca was significantly increased by MIN and CM treatment in FC (p < 0.001) and by MIN treatment in CC (p = 0.008) soil.
A Exchangeable Mg, B exchangeable K and C exchangeable Ca grouped by soil (FC, CC) after 42 days of incubation with the different treatments: control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM). Data shown are the mean of three replicates; error bars indicate one standard errors of the mean; different letters indicate a significant difference for p < 0.05 and are based on one-way ANOVA with p < 0.05 HSD Tukey’s test
Principal Component Analysis (PCA)
Principal Component Analysis (PCA) (Fig. 7) was performed on soil enzymatic activities, extractable organic C and N, pH, EC, and exchangeable Mg, Ca and K. Each treatment clustered along principal components (PC) that together accounted for 72.5% of the explained variance with 54.6% from PC1 and 17.9% from PC2. A net separation between FC and CC was also observed. Two-way ANOVA performed on the PCA scores (Table S3) confirmed that both ‘treatment’ and ‘soil’ factors significantly affected the PC1 and PC2. The interaction among the two factors was also significant in PC1 (p < 0.001) and PC2 (0.025).
Principal component analysis (PCA) performed on pH, electrical conductivity (EC), exchangeable Mg, K and Ca (Mg_exc, K_exc, Ca_exc), extractable C (Cext), extractable N (Next), microbial biomass C (MBC), acid (PAC) and alkaline (PAL) phosphatases, β-glucosidase (BGLU), tyrosinase (TYR), chitinase (NAG), dehydrogenase (DEHY), and urease (UR) on FC and CC soils. Control (CTR); mineral fertilizer (MIN); dried digestate (DD); digested manure (DM); cattle manure (CM)
Discussion
Digestate Characterization
The INALCA’s plant produced biogas from biomass derived from the beef slaughterhouse by-products, in particular the content of the forestomach and the sludge from the wastewater plant of the factory. The produced digestate was then dehydrated by centrifugation and, subsequently, desiccated producing a dried anaerobic digestate (DD).
As a dried digested, DD exhibited 85.5% of total solids that is at least the double of what reported after dry anaerobic digestion (15–40% TS) and can be advantageous for hygienization, storage and transport [49]. The pH of DD was 7.3 (Table 1), in agreement with the range of 7.3 to 9 depending on the raw material reported by Möller and Müller [15] and Lamolinara et al. [20], a useful pH for a fertilizer. Furthermore, electric conductivity (EC, Table 1) was the lowest in DD in respect to DM and CM and this would prevent soil salinization, since the application of organic amendment can increase soil salinity [50]. Total organic C, total N and C/N ratio were reported to range between 36 and 45% dw, 3 to 14% dw and 3 to 8.5% dw respectively [15], and this fits the characteristics of DD (Table 1). A lower prevalence of ammonium-N was observed in DD (0.6% dw and 0.53% fw) in respect to the range reported by Möller and Müller [15] (1.5–6.8% fw). Studies regarding digested slaughterhouse by-products are few, but Alburquerque et al. [26, 27] reported data from different mixture of ADs with slaughterhouse residues, where C and N concentration reported a different range in respect to the digestate reported by Möller and Müller [15]: TOC was 5.9–5.8 g L−1, Total N was 3.96–2.89 g L−1 mostly as ammonium-N, which was 3.46–2.21 g L−1. The high amount of a liquid fraction would increase soluble ammonium-N and nitrite/nitrate–N when applied to the soil, enforcing the convenience to use a dried material as DD in which about 85% of total N was in organic form (Table 1). Total P resulted higher in DD (2.4% dw) than what reported by Möller and Müller [15] (0.6–1.7% dw) and can be related to the higher total solids, as reported by Lamolinara et al. [20] for different animal manure and slurry. After P fractionation, the total P recovery as sum of fraction was 59.7%, lower than what is reported in literature for organic fertilizers (from 91.4 to 125.7%) and ADs (from 82.4 to 119.2%) with solid digestate exhibiting a less total P recovery [51]. H2O-P and NaHCO3-P are referred as labile fraction, NaOH-P as moderately labile fraction and HCl-P as residual P [52]. The labile P fraction (H2O-P + NaHCO3-P) accounted for about 30% in DD with a major component in H2O soluble fraction (Table S4), and this is in agreement with the fact that most of the available-P (NaHCO3-P) could flow and leach in the liquid fraction [53]. Therefore, the less P recovery could be related to the drying process of DD. The inorganic and organic contaminants were lower than limit reported in the EU Regulation 2019/1009. Consequently, in a preliminary assessment, it can be asserted that DD does not pose inherent risks for the environment and is congruent with the characteristics of other organic fertilizers.
Soil Incubation
Inorganic N and Available P Release
Monitoring nitrogen (N) release in soil after organic fertilization is crucial, as it influences ecosystem functioning and impacts the soil microbial community. In FC a significant increase of nitrite/nitrate–N was reported after MIN, DD and CM treatments already at day 3, whereas in CC it was reported at day 14 only after MIN treatment (Table 2). Several factors affect N mineralization in soil, such as the moisture, temperature, organic matter content and texture [54]. The loamy texture of CC (37% sand) in respect to the sandy-loam texture of FC (60%, Table S1) could have contributed to the oxygen limitation and a slower nitrification in CC soil (Table 2). After 42 days of incubation, about 26% and 10% of inorganic N was released by DD in FC and CC soils respectively, but not statistically significant in CC soil (Table 2). Organic fertilizers release N in a range of 25–76% of total N content depending on the soil characteristics and amendments [55]. In literature, Müller-Stöver et al. [56] and Chiyoka et al. [57] reported an increase of nitrate–N and ammonium-N in soil after fertilization with ADs. However, negative net N release ranging from − 8 to − 96% was reported for cattle and pig slurry-based digestates [26, 27], resulting in N immobilization, which is often associated with AD fertilization [24, 57, 58]. N mineralization is influenced by fertilizer C/N ratio, and C/N > 20 can be associated to N immobilization [59]. The ADs tested by Müller-Stöver et al. [56] showed C/N ranging from 3.2 to 5.2. In this experiment, DM reported a value of C/N of 21.3 (Table 1), and this could have contributed to N immobilization [60]. Conversely, DD reported a C/N of 8 (Table 1) and a positive net N release at least FC soil, that suggest the intrinsic suitability of DD to exploit its N-fertilizing potential in this soil.
Available P (Table 3) was released already at day 0 then slightly decreased in time, mostly in CC soil (Table 3). Such decrease was probably due to the high CaCO3 content (Table S1) which facilitates soluble P precipitation in neutral-sub-alkaline soils [61]. In fact, at the end of the incubation, DD released about 16 and 13% of their total P in FC and CC soils, respectively, about a half of labile-P content (30%, Tables 1; S4). Similar release was observed for CM, whereas DM released about 26 and 27% in FC and CC soil, respectively. Study reported an increase of available P ranging from 3 to 26% after organic fertilization with highest increase after cattle manure application [49]. However, available P release was influenced by the added dose which was calculated on total N and not on total P. Digestates reported different available P-release depending on their nature [22], dose and liquid to solid fractions ratio [24, 62]. Data obtained in this experiment fit the ranges found in the literature for available P-released by organic fertilizers and could suggest a higher presence of recalcitrant P rather than available-Olsen P in DD.
Biochemical Indicators
Soil biochemical properties are highly sensitive to agricultural and nutrient input and were monitored at the last day of the incubation to assess the short-term impact of the treatments on the soil fertility after the release of N and P plateaued. DD, DM and CM treatments had a minor effect on soil Cext in FC soil, whereas increased it in CC soil (Fig. 2A). Effects on Cext can vary depending on the fertilizer and among soils [63]. An increase in Cext in short-term experiments can be attributed to the presence of soluble C-materials in amendments [64]. CM was a non-digested fertilizer with probably a higher level of soluble-C forms which could have increased Cext. Microbial biomass C (MBC) is generally increased after solid digestate application [65] and tend to be the lowest in unfertilized soils or soils treated with mineral fertilizers [66]. The highest increase in MBC was reported for CM and DM treatments (Fig. 2C). It is reviewed that only when high level of soluble C is applied by ADs onto soil MBC is increased for more than few weeks after the fertilization [67]. Moreover, since the dose of each fertilizer was based on total N, C added was different (on average 0.9, 2.1 and 1,2 g/kg for DD, DM and CM respectively). A lower MBC for DD could also reflect the lower added C and the presence of recalcitrant organic C form. This would suggest a higher stability for DD in respect to the other fertilizers, since digestion depletes labile organic C pools [68]. Extractable N (Fig. 2 B) follows the same trend of the day 42 of nitrite/nitrate–N release and confirm the result (Table 2). A major increase of Next was given by MIN and showed an inverse trend of MBC as expected for a mineral fertilizer; DD and CM also increased Next as expected for organic fertilizers. Conversely, DM resulted in immobilization as discussed above.
Generally, organic fertilization have reported to have an effect on microbial biomass activity and therefore reflecting soil biochemical parameters [69]. In short-term incubation studies it was observed that fertilization with different organic wastes present different enzyme activity fingerprint, particularly in the case of bioenergy residues [63, 70]. Common responses of DD in both soils were observed for β-glucosidase and chitinase activities (Fig. 3C, D), which presented the same trend of Cext and Next, respectively (Fig. 2A, B). Acid and alkaline phosphatase activities instead were roughly inversely related to available P as expected [71] and CM increased phosphatases more than DD (Fig. 3A, B). This behaviour could be related to the higher organic C availability in soils treated with CM, as soil phosphatase activity also positively correlates with dissolved organic C [72]. Soil dehydrogenase activity is monitored as an index of energy metabolism and biomass activity [73] and was reported to vary greatly among different biogas residues, generally but not always increasing after the treatment [19]. Dehydrogenase (Fig. 3G) was increased after DD treatment only in CC, as well as Cext. In fact, it is often associated to Cext [24] and strongly dependent on the soil type and properties [73]. Enzymatic activity expressed per unit of MBC (specific enzymatic activity) represents the metabolic status of the microbial community [74,75,76]. Specific enzymatic activities (Table S2) were generally lower in CC soil, suggesting a lower basal enzyme synthesis by soil microorganisms [77] that could explain the slower nitrification in this soil (Table 2). Soil urease activity tended to be increased in FC and decreased in CC after the treatments (Fig. 3F), but this trend is not maintained in specific urease activity that however tended to decrease after the treatments in both soils (Table S4). It is reported that urease can be inhibited by slow release fertilizer for ammonium-N accumulation [78] but the exact mechanisms remain to be clarified [79]. The significant increase in some specific enzyme activities in CM in respect to the other treatments could be related to a greater energetic metabolism in response to manure fertilization [80, 81] (Table S4), [82] that are often associated to a higher Cext content and to a lower nutrient availability [76]. In summary, the highest increase of enzymatic activity was associated to higher dissolved organic C provided by organic fertilizers, whereas DD had a stimulant effect only on chitinase and phosphatase activity related to nutrient (N, P) release.
Community Level Physiological Profiling
Community level physiological profiling (CLPP) provided an analysis of the functional microbial diversity of the soil microbial community after different agricultural practices based on the carbon consumption profiling [83]. Final AWCD values (Fig. 4A) are used as an index of metabolic diversity at the end of the treatment and were not different among treatment in FC; conversely, functional diversity was increased in CC after DD and DM treatments. It is not surprising that MIN exhibited the lowest AWCD values, as mineral fertilizers do not stimulate complex carbon-sources degradation by microbial activity, and functional diversity is strongly impacted by soil organic matter quality [83]. As a confirmation of this, the relative degradation of different groups of substrates such as amines & amides (Am_Am, Fig. 4B) was lower in MIN treatments, as an effect of the reduced microbial diversity by inorganic fertilization [84] whereas DD induced a similar level of substrate degradation to the other fertilizers, supporting the effect of DD on the functional microbial diversity as well as the other organic fertilizers.
Chemical Indicators
Finally, soil samples were dried and analysed for the main chemical indicators. All the treatments decreased pH in both soils (Fig. 5A), as an effect of N mineralization that occur in most N-containing fertilizers [85, 86]. EC increased after the treatments (Fig. 5B), mostly after MIN fertilization and CM, and it is known that manure application increase EC in soil [87, 88]. However, EC values are way below the critical range for plant growth established by Abad et al. [89] of 0.75–3.49 dS m−1. Exchangeable cations are considered an indicator of the soil fertility. DD increased exchangeable Mg (Fig. 6 A) in both soils, similarly of what observed for DM and CM which also have comparable values for total Mg (Table 1). Conversely, the only increase of exchangeable K (Fig. 6 B) was given by DM and CM similarly to the observed higher total K content in DM and CM than in DD (Table 1). The higher increase of exchangeable Ca (Fig. 6 C) was reported by MIN in both soils, probably because of a methodological artifact which would include part of the Ca-P precipitate formed after DAP dissolution in the extracted sample [90]. However, neither DD or DM had significant effect on exchangeable Ca independently by their total Ca content (Table 1). It is known that ratios between nutrients is determinant in soil fertility rather tant their absolute quantity [91], in particular the ratio of K to Mg [92]. Exchangeable K to Mg ratio was about 4 times higher in FC, ranging from 2.1 to 2.3, whereas in CC ranged from 0.5 to 0.7.Generally, values of K/Mg close to 2 which can assimilated to an “ideal soil” [92], whereas CC showed a general basal imbalanced K/Mg ratio. Variations of K to Mg were only observed after DM and CM treatment, but the most determinant variable factor affecting the deviation from the ideal value was the soil. In summary, chemical indicators were barely affected by DD treatment.
Principal Component Analysis (PCA)
To summarize the effect of the different fertilizers on chemical and biochemical indicators, PCA analysis was performed (Fig. 7). The first two principal components (PC1 and PC2) together explained 72.5% of the total variance, which represents a good quality 2-dimensional approximation [93]. Each treatment clustered along PC1 and PC2, but the major diversity between samples was given by the soil factor explained by two-way ANOVA (Table S3). In fact, the two soils were different for their physico-chemical characteristics and for their chemical and biochemical index of fertility (Table S1) and thus responded differently to the treatments. However, each treatment was significantly different among the others in each soil, as well as the interaction of soil and treatment (Table S3). The variables that mostly characterized CC soil were Next, exchangeable Mg (Mgexc), Ca (Caexc), EC and UR, corresponding to many of the indexes that reported a different trend in CC than FC soil for their pattern (UR) or for their absolute values (Next, Mgexc and EC). The presence of excess of N for the slower nitrification rate in CC after the incubation (Table 2) may have affected soil salinity [94]. Control soils clustered toward pH, which is the index that was always impacted (decreased) by treatments conversely to CM which clustered toward MBC, Cext, DEHY, PAC and PAL. DM and DD always showed intermediate clusterization between MIN and CM, which in this evaluation could collocate digestates among mineral and organic fertilizers.
Conclusions
Slaughterhouse by-products showed the potential to be converted in organic fertilizer after anaerobic digestion, raising the concrete possibility to apply DD in the soil as a bio-based fertilizers. In fact, physico-chemical characteristics of DD were in the same range of other digestates and organic fertilizers described in literature. Moreover, none of the possible contaminants exceeded the thresholds indicated by Reg. (EU) 2019/1009, discouraging possible undesirable side-effects on the environment. The short-term incubation experiment revealed that DD was able to exploit its fertilizing potential as a N-P fertilizer, releasing about 26% and 10% of the N added, and about 13 and 16% of P in FC and CC respectively, reflecting the different basal fertility of the two soils. The principal indicators of the soil fertility, such as chitinase and phosphatases activity, reported a modest stimulation. Nevertheless, evidence for the presence of recalcitrant C forms in spite of soluble C could indicate a high stability of slaughterhouse by-products after anaerobic digestion, possibly stimulating the C retention in the soil while activating N and P cycling in the soil. Further perspectives would include field trials to (i) confirm the results obtained in this short-term experiment; (ii) observe the nutrient release kinetics in different pedo-climatic conditions; (iii) confirm the fertilizing potential on the growth of different crops.
Data Availability
The datasets generated and analysed during the current study are available in the AMSacta repository, https://amsacta.unibo.it/id/eprint/7678
Abbreviations
- FC:
-
Fulvic cambisol soil
- CC:
-
Clay-Loam cambisol soil
- CTR:
-
Control
- MIN:
-
Mineral fertilizer
- DD:
-
Dried anaerobic digestate
- DM:
-
Digested manure
- CM:
-
Cattle manure
- TOC:
-
Total organic carbon
- TN:
-
Total nitrogen
- OUR:
-
Oxygen uptake rate
- Next :
-
Extractable nitrogen in K2SO4
- Cext :
-
Extractable carbon in K2SO5
- MBC:
-
Microbial biomass carbon with fumigation-extraction method
- PAC:
-
Acid phosphatase
- PAL:
-
Alkaline phosphatase
- BGLU:
-
Beta glucosidase
- DEHY:
-
Dehydrogenase
- NAG:
-
Chitinase
- TYR:
-
Tyrosinase
- UR:
-
Urease
- NO3-N:
-
Nitrite/nitrate nitrogen
- NH4-N:
-
Ammonium nitrogen
- AWCD 120:
-
Average well colour development after 120 h
- CLPP:
-
Community level physiological profiling
- Carb:
-
Carbohydrates
- Carb_Ket:
-
Caboxylic & ketoacids
- AA:
-
Aminoacids
- Poly:
-
Polymers
- Am_Am:
-
Amines & amides
- Ca_exc:
-
Exchangeable calcium in cobalt hexamine
- Mg_exc:
-
Exchangeable magnesium in cobalt hexamine
- K_exc:
-
Exchangeable calcium in potassium hexamine
- EC:
-
Electric conductivity
- pH:
-
Soil reaction
References
Wagner, P.: The triple problem displacement: climate change and the politics of the great acceleration. Eur. J. Soc. Theory 26(1), 24–47 (2023). https://doi.org/10.1177/13684310221136083
Haque, F., Fan, C., Lee, Y.-Y.: From waste to value: addressing the relevance of waste recovery to agricultural sector in line with circular economy. J. Clean. Prod. 415, 137873 (2023). https://doi.org/10.1016/j.jclepro.2023.137873
Tunn, V.S.C., Bocken, N.M.P., van den Hende, E.A., Schoormans, J.P.L.: Business models for sustainable consumption in the circular economy: an expert study. J. Clean. Prod. 212, 324–333 (2019). https://doi.org/10.1016/j.jclepro.2018.11.290
Nghiem, L.D., Hai, F.I., Price, W.E., Wickham, R., Ngo, H.H., Guo, W.: By-products of anaerobic treatment. In: Current developments in biotechnology and bioengineering, pp. 469–484. Elsevier (2017)
Mikusińska, J., Kuźnia, M., Czerwińska, K., Wilk, M.: Hydrothermal carbonization of digestate produced in the biogas production process. Energies 16(14), 5458 (2023). https://doi.org/10.3390/en16145458
Czekała, W., Jasiński, T., Grzelak, M., Witaszek, K., Dach, J.: Biogas plant operation: digestate as the valuable product. Energies 15(21), 8275 (2022). https://doi.org/10.3390/en15218275
Fagerström, A.: The role of anaerobic digestion and biogas in the circular economy (J. D. Murphy, Ed.). IEA Bioenergy (2018)
Mozhiarasi, V., Natarajan, T.S.: Slaughterhouse and poultry wastes: management practices, feedstocks for renewable energy production, and recovery of value added products. Biomass Convers Biorefinery (2022). https://doi.org/10.1007/s13399-022-02352-0
Struthers Montford, K., Wotherspoon, T.: The contagion of slow violence: the slaughterhouse and COVID-19. Animal Studies Journal 10(1), 80–113 (2021). https://doi.org/10.14453/asj.v10i1.6
Shende, A.D., Pophali, G.R.: Anaerobic treatment of slaughterhouse wastewater: a review. Environ. Sci. Pollut. Res. 28(1), 35–55 (2021). https://doi.org/10.1007/s11356-020-10921-x
Reyes, I.P., Díaz, J.P., Horváth, I.S.: Anaerobic biodegradation of solid substrates from agroindustrial activities—slaughterhouse wastes and agrowastes. In: Chamy, R., Rosenkranz, F., Soler, L. (eds.) Biodegradation and bioremediation of polluted systems—new advances and technologies. InTech (2015)
Ware, A., Power, N.: Biogas from cattle slaughterhouse waste: Energy recovery towards an energy self-sufficient industry in Ireland. Renewable Energy 97, 541–549 (2016). https://doi.org/10.1016/j.renene.2016.05.068
FAO: Soils for nutrition: state of the art. FAO (2022). https://doi.org/10.4060/cc0900en
Breure, A.M., Lijzen, J.P.A., Maring, L.: Soil and land management in a circular economy. Sci. Total. Environ. 624, 1125–1130 (2018). https://doi.org/10.1016/j.scitotenv.2017.12.137
Möller, K., Müller, T.: Effects of anaerobic digestion on digestate nutrient availability and crop growth: a review—digestate nutrient availability. Eng. Life Sci. 12(3), 242–257 (2012). https://doi.org/10.1002/elsc.201100085
Pastorelli, R., Valboa, G., Lagomarsino, A., Fabiani, A., Simoncini, S., Zaghi, M., Vignozzi, N.: Recycling biogas digestate from energy crops: effects on soil properties and crop productivity. Appl. Sci. 11(2), 750 (2021). https://doi.org/10.3390/app11020750
Cesaro, A.: The valorization of the anaerobic digestate from the organic fractions of municipal solid waste: challenges and perspectives. J. Environ. Manage. 280, 111742 (2021). https://doi.org/10.1016/j.jenvman.2020.111742
Lencioni, G., Imperiale, D., Cavirani, N., Marmiroli, N., Marmiroli, M.: Environmental application and phytotoxicity of anaerobic digestate from pig farming by in vitro and in vivo trials. Int. J. Environ. Sci. Technol. 13(11), 2549–2560 (2016). https://doi.org/10.1007/s13762-016-1088-y
Nielsen, K., Roß, C.-L., Hoffmann, M., Muskolus, A., Ellmer, F., Kautz, T.: The chemical composition of biogas digestates determines their effect on soil microbial activity. Agriculture 10(6), 244 (2020). https://doi.org/10.3390/agriculture10060244
Lamolinara, B., Pérez-Martínez, A., Guardado-Yordi, E., Guillén Fiallos, C., Diéguez-Santana, K., Ruiz-Mercado, G.J.: Anaerobic digestate management, environmental impacts, and techno-economic challenges. Waste Manage. 140, 14–30 (2022). https://doi.org/10.1016/j.wasman.2021.12.035
Möller, K.: Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity: a review. Agron. Sustain. Dev. 35(3), 1021–1041 (2015). https://doi.org/10.1007/s13593-015-0284-3
Barłóg, P., Hlisnikovský, L., Kunzová, E.: Effect of digestate on soil organic carbon and plant-available nutrient content compared to cattle slurry and mineral fertilization. Agronomy 10(3), 379 (2020). https://doi.org/10.3390/agronomy10030379
Seleiman, M.F., Selim, S., Jaakkola, S., Mäkelä, P.S.A.: Chemical composition and in vitro digestibility of whole-crop maize fertilized with synthetic fertilizer or digestate and harvested at two maturity stages in Boreal growing conditions. Agric. Food Sci. 26(1), 47 (2017). https://doi.org/10.23986/afsci.60068
Valentinuzzi, F., Cavani, L., Porfido, C., Terzano, R., Pii, Y., Cesco, S., Marzadori, C., Mimmo, T.: The fertilising potential of manure-based biogas fermentation residues: pelleted vs. liquid digestate. Heliyon 6(2), e03325 (2020). https://doi.org/10.1016/j.heliyon.2020.e03325
Alba-Reyes, Y., Hermida-García, F.O., Pedraza-Garciga, J., López-González, L.M., Espinosa-Negrín, A.M., Carbonell-Sorí, L., Barrera, E.L.: Economic and environmental assessment of a biogas-based pressurized grid in a livestock farm: a case study in a cuban context. J. Clean. Prod. 434, 140288 (2024). https://doi.org/10.1016/j.jclepro.2023.140288
Alburquerque, J.A., De La Fuente, C., Bernal, M.P.: Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agr Ecosyst Environ 160, 15–22 (2012). https://doi.org/10.1016/j.agee.2011.03.007
Alburquerque, J.A., de la Fuente, C., Ferrer-Costa, A., Carrasco, L., Cegarra, J., Abad, M., Bernal, M.P.: Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenerg. 40, 181–189 (2012). https://doi.org/10.1016/j.biombioe.2012.02.018
Ervasti, S., Kostensalo, J., Tampio, E.: Effects of seasonal and local co-feedstocks on the performance of continuous anaerobic digestion of cattle slurry. Bioresour Technol Rep 19, 101207 (2022). https://doi.org/10.1016/j.biteb.2022.101207
Zhang, Y., Banks, C.J.: Co-digestion of the mechanically recovered organic fraction of municipal solid waste with slaughterhouse wastes. Biochem. Eng. J. 68, 129–137 (2012). https://doi.org/10.1016/j.bej.2012.07.017
Dick, W.A., Culman, S.W.: Biological and biochemical tests for assessing soil fertility. In: Chatterjee, A., Clay, D. (eds.) ASA, CSSA, and SSSA books, pp. 134–147. American Society of Agronomy and Soil Science Society of America (2017)
Teglia, C., Tremier, A., Martel, J.-L.: Characterization of solid digestates: part 1, review of existing indicators to assess solid digestates agricultural use. Waste Biomass Valorization 2(1), 43–58 (2011). https://doi.org/10.1007/s12649-010-9051-5
Ciavatta, C., Antisari, L.V., Sequi, P.: Determination of organic carbon in soils and fertilizers. Commun. Soil Sci. Plant Anal. 20(7–8), 759–773 (1989). https://doi.org/10.1080/00103628909368115
A.O.A.C. (1990). Official methods of analysis (Vol. 1). ISBN: 0-935584-42-0
U.S. EPA. (2006). Method 1682: Salmonella in sewage sludge (biosolids) by modified semisolid Rappaport-Vassiliadis (MSRV) medium. EPA-821-R-06-14
Dinkler, K., Li, B., Guo, J., Hülsemann, B., Becker, G.C., Müller, J., Oechsner, H.: Adapted Hedley fractionation for the analysis of inorganic phosphate in biogas digestate. Biores. Technol. 331, 125038 (2021). https://doi.org/10.1016/j.biortech.2021.125038
Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnson, C.T., Sumner, M.E.: Methods of soil analysis: Part 3, chemical methods. Soil Science Society of America, American Society of Agronomy (1996)
Olsen, S.R., Cole, C.V., Watanabe, F.S., Dean, L.A. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Circular 939, U. S. Department of Agriculture (1954)
Vance, E.D., Brookes, P.C., Jenkinson, D.S.: An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 19(6), 703–707 (1987). https://doi.org/10.1016/0038-0717(87)90052-6
Eivazi, F., Tabatabai, M.A.: Phosphatases in soils. Soil Biol. Biochem. 9(3), 167–172 (1977). https://doi.org/10.1016/0038-0717(77)90070-0
Eivazi, F., Tabatabai, M.A.: Glucosidases and galactosidases in soils. Soil Biol. Biochem. 20(5), 601–606 (1988). https://doi.org/10.1016/0038-0717(88)90141-1
Parham, J.A., Deng, S.P.: Detection, quantification and characterization of B- glucosaminidase activity in soil. Soil Biol 32, 1183–1190 (2000)
Kandeler, E., Gerber, H.: Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertility Soils (1988). https://doi.org/10.1007/BF00257924
Sinsabaugh, R.L., Klug, M.J., Yeager, H.P., Peterson, S.O.: Characterizing soil microbial communities, standard soil methods for long-term ecological research. Oxford University Press, New York (1999)
von Mersi, W., Schinner, F.: An improved and accurate method for determining the dehydrogenase activity of soils with iodonitrotetrazolium chloride. Biol. Fertil. Soils 11(3), 216–220 (1991). https://doi.org/10.1007/BF00335770
Gryta, A., Frąc, M., Oszust, K.: The application of the biolog ecoplate approach in ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 174(4), 1434–1443 (2014). https://doi.org/10.1007/s12010-014-1131-8
R Core Team. R: a language and environment for statistical computing [Computer software] (2022)
Ge, Z., Du, H., Gao, Y., Qiu, W.: Analysis on metabolic functions of stored rice microbial communities by BIOLOG ECO microplates. Front. Microbiol. 9, 1375 (2018). https://doi.org/10.3389/fmicb.2018.01375
Weber, K.P., Legge, R.L.: Community-level physiological profiling. In: Cummings, S.P. (ed.) Bioremediation, vol. 599, pp. 263–281. Humana Press (2010)
Wang, Z., Hu, Y., Wang, S., Wu, G., Zhan, X.: A critical review on dry anaerobic digestion of organic waste: characteristics, operational conditions, and improvement strategies. Renew. Sustain. Energy Rev. 176, 113208 (2023). https://doi.org/10.1016/j.rser.2023.113208
Eneji, A.E., Honna, T., Yamamoto, S.: Manuring effect on rice grain yield and extractable trace elements in soils. J. Plant Nutr. 24(7), 967–977 (2001). https://doi.org/10.1081/PLN-100103797
Brod, E., Øgaard, A.F., Hansen, E., Wragg, D., Haraldsen, T.K., Krogstad, T.: Waste products as alternative phosphorus fertilisers part I: inorganic P species affect fertilisation effects depending on soil pH. Nutr. Cycl. Agroecosyst. 103(2), 167–185 (2015). https://doi.org/10.1007/s10705-015-9734-1
Negassa, W., Leinweber, P.: How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: a review. J. Plant Nutr. Soil Sci. 172(3), 305–325 (2009). https://doi.org/10.1002/jpln.200800223
Mazzini, S., Borgonovo, G., Scaglioni, L., Bedussi, F., D’Imporzano, G., Tambone, F., Adani, F.: Phosphorus speciation during anaerobic digestion and subsequent solid/liquid separation. Sci. Total. Environ. 734, 139284 (2020). https://doi.org/10.1016/j.scitotenv.2020.139284
Robertson, G.P., Groffman, P.M.: Nitrogen transformations. In: Soil microbiology, ecology and biochemistry, pp. 421–446. Elsevier (2015)
Uddin, S., Islam, M.R., Jahangir, M.M.R., Rahman, M.M., Hassan, S., Hassan, M.M., Abo-Shosha, A.A., Ahmed, A.F., Rahman, M.M.: Nitrogen release in soils amended with different organic and inorganic fertilizers under contrasting moisture regimes: a laboratory incubation study. Agronomy 11(11), 2163 (2021). https://doi.org/10.3390/agronomy11112163
Müller-Stöver, D.S., Sun, G., Kroff, P., Thomsen, S.T., Hauggaard-Nielsen, H.: Anaerobic co-digestion of perennials: methane potential and digestate nitrogen fertilizer value. J. Plant Nutr. Soil Sci. 179, 696–704 (2016). https://doi.org/10.1002/jpln.201500599
Chiyoka, W.L., Zvomuya, F., Hao, X.: Changes in nitrogen availability in chernozemic soils amended with anaerobically digested cattle manure. Soil Sci. Soc. Am. J. 78(3), 843–851 (2014). https://doi.org/10.2136/sssaj2013.07.0297
Pantelopoulos, A., Magid, J., Jensen, L.S.: Net and gross nitrogen turnover in soil amended with acidified and differently dried solids from biogas digestate. Soil Sci. Soc. Am. J. 80(4), 943–953 (2016). https://doi.org/10.2136/sssaj2016.03.0059
Cordovil, C.M.D.S., Coutinho, J., Goss, M., Cabral, F.: Potentially mineralizable nitrogen from organic materials applied to a sandy soil: fitting the one-pool exponential model. Soil Use Manag. 21(1), 65–72 (2006). https://doi.org/10.1111/j.1475-2743.2005.tb00108.x
Enwezor, W.O.: The mineralization of nitrogen and phosphorus in organic materials of varying C: N and C: P ratios. Plant Soil 44(1), 237–240 (1976). https://doi.org/10.1007/BF00016972
Fan, X.H., Li, Y.C.: Nitrogen release from slow-release fertilizers as affected by soil type and temperature. Soil Sci. Soc. Am. J. 74(5), 1635–1641 (2010). https://doi.org/10.2136/sssaj2008.0363
Tambone, F., Orzi, V., D’Imporzano, G., Adani, F.: Solid and liquid fractionation of digestate: Mass balance, chemical characterization, and agronomic and environmental value. Biores. Technol. 243, 1251–1256 (2017). https://doi.org/10.1016/j.biortech.2017.07.130
Galvez, A., Sinicco, T., Cayuela, M.L., Mingorance, M.D., Fornasier, F., Mondini, C.: Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agr Ecosyst Environ 160, 3–14 (2012). https://doi.org/10.1016/j.agee.2011.06.015
Chantigny, M.H.: Dissolved and water-extractable organic matter in soils: a review on the influence of land use and management practices. Geoderma 113(3–4), 357–380 (2003). https://doi.org/10.1016/S0016-7061(02)00370-1
Cattin, M., Semple, K.T., Stutter, M., Romano, G., Lag-Brotons, A.J., Parry, C., Surridge, B.W.J.: Changes in microbial utilization and fate of soil carbon following the addition of different fractions of anaerobic digestate to soils. Eur. J. Soil Sci. 72(6), 2398–2413 (2021). https://doi.org/10.1111/ejss.13091
Kaur, K., Kapoor, K.K., Gupta, A.P.: Impact of organic manures with and without mineral fertilizers on soil chemical and biological properties under tropical conditions. J. Plant Nutr. Soil Sci. 168(1), 117–122 (2005). https://doi.org/10.1002/jpln.200421442
Van Midden, C., Harris, J., Shaw, L., Sizmur, T., Pawlett, M.: The impact of anaerobic digestate on soil life: a review. Appl. Soil Ecol. 191, 105066 (2023). https://doi.org/10.1016/j.apsoil.2023.105066
Terhoeven-Urselmans, T., Scheller, E., Raubuch, M., Ludwig, B., Joergensen, R.G.: CO2 evolution and N mineralization after biogas slurry application in the field and its yield effects on spring barley. Appl. Soil Ecol. 42(3), 297–302 (2009). https://doi.org/10.1016/j.apsoil.2009.05.012
Nkoa, R.: Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: a review. Agron. Sustain. Dev. 34(2), 473–492 (2014). https://doi.org/10.1007/s13593-013-0196-z
Stumpe, B., Werner, S., Jung, R., Heinze, S., Jüschke, E., Strippel, C., Marschner, B.: Organic carbon dynamics and enzyme activities in agricultural soils amended with biogas slurry, liquid manure and sewage sludge. Agric. Sci. 03(01), 104–113 (2012). https://doi.org/10.4236/as.2012.31014
Criquet, S., Braud, A.: Effects of organic and mineral amendments on available P and phosphatase activities in a degraded Mediterranean soil under short-term incubation experiment. Soil Tillage Res 98(2), 164–174 (2008). https://doi.org/10.1016/j.still.2007.11.001
Kouchou, A., Rais, N., Thoisy, J.-C., Duplay, J., Ghazi, M., Elsass, F., Ijjaali, M., El Ghachtouli, N.: Behavior of enzyme activities exposed to contamination by heavy metals and dissolved organic carbon in calcareous agricultural soils. Soil Sediment Contamin 26(3), 259–276 (2017). https://doi.org/10.1080/15320383.2017.1289499
Beyer, L., Wachendorf, C., Elsner, D.C., Knabe, R.: Suitability of dehydrogenase activity assay as an index of soil biological activity. Biol. Fertil. Soils 16(1), 52–56 (1993). https://doi.org/10.1007/BF00336515
Landi, L., Renella, G., Moreno, J.L., Falchini, L., Nannipieri, P.: Influence of cadmium on the metabolic quotient, l -: D -glutamic acid respiration ratio and enzyme activity: microbial biomass ratio under laboratory conditions. Biol. Fertil. Soils 32(1), 8–16 (2000). https://doi.org/10.1007/s003740000205
Nsabimana, D., Haynes, R.J., Wallis, F.M.: Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl. Soil Ecol. 26(2), 81–92 (2004). https://doi.org/10.1016/j.apsoil.2003.12.005
Raiesi, F., Beheshti, A.: Soil specific enzyme activity shows more clearly soil responses to paddy rice cultivation than absolute enzyme activity in primary forests of northwest Iran. Appl. Soil Ecol. 75, 63–70 (2014). https://doi.org/10.1016/j.apsoil.2013.10.012
Bastida, F., Zsolnay, A., Hernández, T., García, C.: Past, present and future of soil quality indices: a biological perspective. Geoderma 147(3–4), 159–171 (2008). https://doi.org/10.1016/j.geoderma.2008.08.007
Jiao, X., Liang, W., Chen, L., Zhang, H., Li, Q., Wang, P., Wen, D.: Effects of slow-release urea fertilizers on urease activity, microbial biomass, and nematode communities in an aquic brown soil. Sci. China, Ser. C Life Sci. 48(S1), 26–32 (2005). https://doi.org/10.1007/BF02889798
Koçak, B. (2020). Importance of urease activity in soil. ISBN: 978-605-74786-0-3
Bell, J.M., Robinson, C.A., Schwartz, R.C.: Changes in soil properties and enzymatic activities following manure applications to a rangeland. Rangel. Ecol. Manage. 59(3), 314–320 (2006). https://doi.org/10.2111/05-172R1.1
Liang, Q., Chen, H., Gong, Y., Yang, H., Fan, M., Kuzyakov, Y.: Effects of 15 years of manure and mineral fertilizers on enzyme activities in particle-size fractions in a North China Plain soil. Eur. J. Soil Biol. 60, 112–119 (2014). https://doi.org/10.1016/j.ejsobi.2013.11.009
Liu, S., Razavi, B.S., Su, X., Maharjan, M., Zarebanadkouki, M., Blagodatskaya, E., Kuzyakov, Y.: Spatio-temporal patterns of enzyme activities after manure application reflect mechanisms of niche differentiation between plants and microorganisms. Soil Biol. Biochem. 112, 100–109 (2017). https://doi.org/10.1016/j.soilbio.2017.05.006
Bending, G.D., Turner, M.K., Jones, J.E.: Interactions between crop residue and soil organic matter quality and the functional diversity of soil microbial communities. Soil Biol. Biochem. 34(8), 1073–1082 (2002). https://doi.org/10.1016/S0038-0717(02)00040-8
Zhang, Q.-C., Shamsi, I.H., Xu, D.-T., Wang, G.-H., Lin, X.-Y., Jilani, G., Hussain, N., Chaudhry, A.N.: Chemical fertilizer and organic manure inputs in soil exhibit a vice versa pattern of microbial community structure. Appl. Soil Ecol. 57, 1–8 (2012). https://doi.org/10.1016/j.apsoil.2012.02.012
Dong, Y., Yang, J.-L., Zhao, X.-R., Yang, S.-H., Mulder, J., Dörsch, P., Zhang, G.-L.: Seasonal dynamics of soil pH and N transformation as affected by N fertilization in subtropical China: an in situ 15N labeling study. Sci. Total. Environ. 816, 151596 (2022). https://doi.org/10.1016/j.scitotenv.2021.151596
Liu, E., Yan, C., Mei, X., He, W., Bing, S.H., Ding, L., Liu, Q., Liu, S., Fan, T.: Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 158(3–4), 173–180 (2010). https://doi.org/10.1016/j.geoderma.2010.04.029
Azeez, J.O., Van Averbeke, W.: Dynamics of soil pH and electrical conductivity with the application of three animal manures. Commun. Soil Sci. Plant Anal. 43(6), 865–874 (2012). https://doi.org/10.1080/00103624.2012.653022
Roy, S., Kashem, Md.A.: Effects of organic manures in changes of some soil properties at different incubation periods. Open J Soil Sci 04(03), 81–86 (2014). https://doi.org/10.4236/ojss.2014.43011
Abad, M., Noguera, P., Burés, S.: National inventory of organic wastes for use as growing media for ornamental potted plant production: case study in Spain. Biores. Technol. 77(2), 197–200 (2001). https://doi.org/10.1016/S0960-8524(00)00152-8
Ernani, P.R., Barber, S.A.: Comparison of P- availability from monocalcium and diammonium phosphates using a mechanistic nutrient uptake model. Fertilizer Res 22(1), 15–20 (1990). https://doi.org/10.1007/BF01054802
Bear, F.F., Prince, A.L., Toth, S.J., Purvin, E.R.: Magnesium in plants and soil. NJ Agric. Bull. 760 (1951)
Kopittke, P.M., Menzies, N.W.: A review of the use of the basic cation saturation ratio and the “ideal” soil. Soil Sci. Soc. Am. J. 71(2), 259–265 (2007). https://doi.org/10.2136/sssaj2006.0186
Jolliffe, I.T., Cadima, J.: Principal component analysis: a review and recent developments. Phil. Trans. R. Soc. A 374(2065), 20150202 (2016). https://doi.org/10.1098/rsta.2015.0202
Han, J., Shi, J., Zeng, L., Xu, J., Wu, L.: Effects of nitrogen fertilization on the acidity and salinity of greenhouse soils. Environ. Sci. Pollut. Res. 22(4), 2976–2986 (2015). https://doi.org/10.1007/s11356-014-3542-z
Acknowledgements
The technical support in the laboratory provided by Paola Gioacchini, Maurizio Quartieri and Andrea Simoni is acknowledged.
Funding
Open access funding provided by Alma Mater Studiorum - Università di Bologna within the CRUI-CARE Agreement. This research was financially supported by EIT FOOD “Sustainable fertilizer from beef slaughtering biogas digested sludge. Digested sludge upgrading and converting into a recognised fertilizer” (project number 21173).
Author information
Authors and Affiliations
Contributions
AC: Investigation, Formal analysis, Metodology, Writing—original draft preparation, Writing—review & editing; GDB: Investigation, Metodology; MR: Resources; MG: Conceptualization, Writing—review & editing; CC: Project administration, Writing—review & editing, Funding acquisition; LC: Conceptualization, Project administration, Metodology, Supervision, Validation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ciurli, A., Di Biase, G., Rossi, M. et al. Dried Anaerobic Digestate from Slaughterhouse By-products: Emerging Cues for a Bio-Based Fertilization. Waste Biomass Valor (2024). https://doi.org/10.1007/s12649-024-02737-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12649-024-02737-4