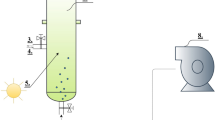
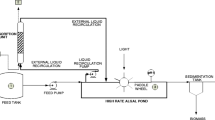
Abstract
The performance of an outdoor pilot-scale system consisting of a high rate algal pond interconnected to an absorption bubble column and seeded with a microalgal-bacterial consortium is presented. Biogas and organic leachate (from food waste anaerobic hydrolysis) were used as sources of CO2 and nutrients for the consortium, respectively. The removal efficiencies for CO2 and H2S from biogas were 80.0% and 99.9%, respectively, provoking a methane content in the utilized biogas of approximately 55 vol% for anaerobically-produced biogas and 90 vol% for a prepared gaseous mixture. The molecular oxygen produced by microalgae photosynthesis induced undesirable cumulation of O2 in utilized biogas up to 5%vol. The organic leachate was diluted in an alkaline mineral medium (15:1 v/v), and this rich-nutrient mixture was fed to the system at a hydraulic retention time of 138 d. The biomass productivity applying organic leachate and anaerobically-produced biogas was 2.6 g m−3 d−1, nine times lower than biomass productivity achieved when only mineral salt medium was fed, suggesting the existence of limited nutrient bioavailability. Microalgal-bacterial community shifts evaluated through molecular and morphological methods showed that a robust and stable photosynthetic population was maintained throughout the entire operation of the system, formed mainly by Picochlorum sp., Pseudanabaena sp., Spirulina sp., and Nitzschia sp., as well as heterotrophic bacteria and some microalgae predators. The valued by-products after waste utilization were clean and highly calorific biogas besides microalgae biomass, where the commercialization of the latter would determine the economic viability of biogas and leachate utilization.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
A microalgae-based valorization system was deployed for biogas upgrading and biomass production under alkaline conditions, it recovered CO2 from biogas and nutrients contained in organic leachate through microalgae assimilation under a scenario of university installations. The system can produce biomethane and microalgae biomass for possible commercialization.
Introduction
The global biogas market was valued at USD 62.72 billion in 2022, with an expected annual growth rate of 4.3% from 2023 to 2030 [1] Nonetheless, this market can increase due to the high fluctuations of international oil prices [2]; however, the worldwide consumption subsidies to fossil fuels are still high, reaching above USD 1 trillion in 2022 [3]. In this context, the generation of biogas from the anaerobic digestion (AD) of organic fraction of municipal solid waste (OFMSW) [4] is desirable to reduce dependency on fossil fuels, where one lucrative option could be biogas in vehicle fuel applications [1]. Marousek et al. [5] indicated that the bioavailability of C and N in OFMSW is a crucial parameter to improve biogas production; therefore, after its upgrading, biogas can be valorized as biomethane [6]. Biogas is typically composed of CH4 (50–70%vol), CO2 (30–45%vol), H2S (0-10k ppmv), nitrogen (0–15%vol), water (1–5%vol) and ammonia (0–100 ppmv) [7, 8]; the concentration of each component depends on the type of organic matter digested to produce this gaseous mixture. CO2 and H2S content must be removed for a clean, highly calorific non-fossil fuel called biomethane [7]. In addition, the OFMSW anaerobic hydrolysis produces a liquid waste generated by percolation called organic leachate characterized by containing COD of around 500020,000 mg L−1 (BOD/COD ranging from 0.4 to 0.7), nitrogen (3000–5000 mg L−1 N-NH3 or N-NH4+), among other dissolved solids (TDS) as well as a high moisture content ranging from 66 to 80% weight and low pH values around 4.0 [9, 10].
In microalgae-bacterial systems, CO2 removal from biogas (or biogas upgrading) is performed by its absorption and consequent fixation in microalgae cells through photosynthesis [11]. Hydrogen sulfide (H2S) is also absorbed and then biologically converted to sulfate (SO42−) by sulfur-oxidizing bacteria using the O2 produced by photosynthesis [6]. In addition, microalgae and microalgal-bacterial consortia have been successfully applied for nutrient removal from different wastewater types like domestic, leachate, agricultural, and industrial [12]. Organic leachate is a promising feedstock to promote the growth of valued microalgae biomass and thus decrease the operational cost associated with the supplying of nutrients since microalgae can assimilate different forms of nitrogen such as nitrate (N-NO3−), urea, and ammonia (N-NH4+) [13]. Nonetheless, it is critical to find an adequate dilution to boost microalgal biomass productivity and reduce the components that can cause their inhibition [14].
Different photobioreactor configurations have been used in open or closed high-rate algal ponds [15, 16]. Similarly, several operational conditions have been evaluated, such as hydraulic retention time [17], nutrient and biogas supply strategies [18], illumination and time cycles (light/dark) [19, 20], as well as increased alkalinity to enhance CO2 mass transfer [21, 22] and changes in liquid /biogas ratio in a split absorption column [23].
Residual microalgal biomass left from the process is a valued byproduct due to its biochemical composition (high protein, lipids, or carbohydrate content) [24] this added value calls for monitoring and identification of the prominent members of the microbial community during their cultivation in open photobioreactors [25] so it is important to control and select an adequate stage to grow the convenient microbial populations. Therefore, the microalgae-based valorization process of organic wastes could have economic advantages in the frame of sustainable development because microalgae biomass can be monetized through various avenues [26]; the cultivation of microalgae can sequester CO2 from biogas, contributing to greenhouse gas reduction; therefore open the option to access to carbon credits [27] if organic leachate is used as a growth medium for microalgae the costs associated with its disposal can be significantly reduced or even eliminated [28]. Biogas produced from the anaerobic digestion of organic waste is commonly used for energy generation [7], and cultivating microalgae under alkaline conditions may require less pH adjustment, reducing the cost of chemicals used for pH control [6].
At the moment, several experiences of organic wastes valorization using microalgae have been carried out mainly at bench-scale with different parameters under control or simulated, where the performance of a less-controlled outdoor pilot-scale High Rate Algal Pond (HRAP) seeded with a microalgal-bacterial consortium and fed with real wastes is scarcely reported. This work aimed to characterize the performance of an outdoor algal-bacterial photobioreactor at a pilot scale during 400 days of operation and deployed for biogas upgrading and nutrients removal from organic leachate produced from anaerobic hydrolysis of the organic fraction of municipal solid waste. It was assumed that the nutrients that remained in organic leachate were bioavailable enough for their microalgae assimilation under the culture conditions. Shifts of microbial communities were assessed during the experimentation; some insights about the viability of this organic waste valorization process are also shown.
Materials and Methods
Experimental Setup and Operational Conditions
The inoculum for the pilot-scale microalgae cultivation system was composed of an alkaliphilic microalgal consortium (AMC) and alkaliphilic sulfur-oxidizing bacteria (ASB) [29]. The pilot-scale microalgae cultivation system consisted of a High Rate Algal Pond (HRAP) (length = 3 m; width = 0.6 m; depth = 16 cm) with a working volume of 300 L and an illuminated area of 1.8 m2. The HRAP was continuously mixed by a stainless steel four-wheel paddle, inducing a liquid velocity of 20 cm s−1 [30]. This system was interconnected to an absorption bubble column (ABC) of 3.2 L (height = 1.0 m; internal diameter = 0.065 m) via an external liquid recirculation stream using a peristaltic pump (model 07559-10, Cole-Parmer®, USA) (Fig. 1). The cultivation broth was fed in a co-current flow with a biogas stream bubbled into the ABC.
The pilot-scale HRAP-ABC system was installed outdoors, next to the wastewater and OFMSW treatment plant at the Universidad Autonoma Metropolitana in Iztapalapa, Mexico City. Two different types of biogas were used (Fig. 1); a commercially prepared gaseous mixture with a composition (%vol) of CH4 (69.5), CO2 (30), and H2S (0.5) (Praxair, Mexico) and an in situ anaerobically-produced biogas from a pilot-scale anaerobic digester of municipal wastewater with a composition (%vol) of CH4 (51.8 ± 10.7), CO2 (4.4 ± 1.2), O2 (6.9 ± 2.5) and N2 (36.6 ± 9.3), and H2S (< 1 ppmv).
Operational Conditions
The pilot-scale HRAP-ABC system was initially filled with mineral salt medium (MSM) composed of (g L−1): Na2CO3 (4.03), NaHCO3 (13.61), NaCl (1.0), K2HPO4 (1.0), K2SO4 (1.0), CaCl2·H2O (0.04), KNO3 (2.52), MgCl2·6H2O (0.2) and 2 ml L−1 of trace elements [22]. This media was then inoculated with 30 L of a microbial cell suspension obtained from a bench-scale HRAP [21], resulting in an initial TSS of 0.3 ± 0.0 g L−1. Table 1 shows the operational mode employed during the experimentation.
At the startup (stage I), the HRAP-ABC system was operated in batch mode for 21 days. Afterward, from day 22 to 36 (stage II), it was changed to continuous mode with a hydraulic retention time (HRT) of 46 d (inlet flow of 6.6 L d−1) by feeding a blend of MSM and leachate (15:1 v/v) with a resulting composition of (g L−1): total organic carbon (2.1), inorganic carbon (1.4) and total nitrogen (0.1) and a final pH of 9.3. The OFMSW was harvested daily at the university cafeteria, milled to 0.7 cm particle diameter, and fed to an anaerobic hydrolysis leaching bed reactor to produce leachate rich in fermentation products. The leachate had a pH of 4.3 ± 0.3 and composition (g L−1) of COD (49 ± 14), CODs (30 ± 6), TS (20 ± 6); VS (13 ± 5); VFACOD (33.3.0 ± 9), KN (1.5 ± 0.3), NH4-N (0.7 ± 0.1), TP (0.24 ± 0.7) [31]. The leachate was used as nutrient feedstock for the pilot-scale cultivation system.
During stages I and II, an airstream was fed to the ABC; meanwhile, at stages III and IV, only a prepared gaseous mixture was introduced to the ABC, attaining an Empty Bed Residence Time (EBRT = ABC volume/gaseous flow rate) of 23 min (Table 1). At stages III and IV, liquid and gas volumetric ratios (L/G) of 0.5 and 1.0 were assayed, respectively. During stage III (from day 250 to 262), a prepared gaseous mixture flow of 100 L d− 1 was established to get an L/G ratio of 0.5, while stage IV (from day 263–287), 200 L d−1 was set to get an L/G ratio of 1.0. Finally, at stage V (from day 293 to 389), the in situ anaerobically-produced biogas was fed in the ABC with a flow of 200 L d−1 (attaining an L/G of 1.0). Samples of 30 mL were taken daily from the pilot HRAP to assess biomass, total nitrogen (TN), inorganic carbon (IC), and total carbon (TC) concentrations. Dissolved oxygen (DO) concentration, pH, and solar irradiance were continuously measured and recorded online every 15 min.
Analytical Procedures
The total suspended solids (TSS) concentration was estimated to equal the microalgal biomass concentration [32]. The dissolved oxygen (DO) concentration and pH were measured by a DO probe (Applisens Z10023525, Applikon, The Netherlands) and a pH electrode (Orion 9107BNMD electrode, Thermo Scientific, USA). The temperature was measured with a thermocouple submerged in the HRAP culture. TC, IC, and TN concentrations were analyzed in a TOCL-L CSH analyzer coupled to a TNM-L chemiluminescence module (Shimadzu, Japan). The gaseous concentrations of CH4, CO2, O2, and H2S at the inlet and the outlet of the ABC were measured from a simple sample collected in a 5 L Tedlar bag; to obtain the values, a portable gas analyzer Biogas 5000 (Geotech, USA) extracted the gas from the bag. The irradiance was measured by an LI-250 A light meter (LI-COR Biosciences, USA) to quantify the Photosynthetically Active Radiation (PAR).
Morphological Microalgae Identification
The morphological identification was performed from an aqueous cell suspension sample collected from the pilot-scale HRAP. A volume of 25 µL of the aqueous cell suspension was screened by an optical microscope at 40X magnification (LSM-800, Carl Zeiss, Germany) coupled to a digital camera (Axiocam 506 color, Carl Zeiss, Germany). The images were digitized with the Zen 2.0 Zeiss software.
DNA Extraction and High-Throughput Sequencing
Several discrete aqueous cell suspension samples were taken from the pilot-scale HRAP-ABC system for amplicon-based sequencing. The samples were taken from the influent (S0), the startup of stage I (SI), the end of stage IV (SIV), and the end of stage V (SV). Approximately 2.5 mg of biomass was collected after centrifugation of the aqueous cell suspension sample for DNA extraction, which was made using the ZR Soil Microbe DNA Kit™. The extracted DNA was stored at − 20 °C before being sent to Macrogen Inc., South Korea, for sequencing. 16 S rRNA and 18 S rRNA genes characterized bacterial and eukaryotic communities. 16 S rRNA and 18 S rRNA amplicon libraries were created and sequenced using an Illumina MiSeq (Illumina) platform. The primers 341 F and 805R were used for 16 S rRNA [33] and 616 F and 1132R for 18 S rRNA [34]. Macrogen Inc. returned the raw data to perform the bioinformatic analyses.
Bioinformatic Analyses
Raw sequences were processed independently for 16 S rRNA and 18 S rRNA genes using QIIME2 [35]. After a quality graphical analysis of the sequences, it was observed that less than 25% of the base pairs had a low-quality score (q < 15). Therefore, the sequences without truncation were used. The DADA2 plug-in with standard parameters for sample inference and chimera filtering was used [36]. The resulting Amplicon Sequence Variants (ASVs) were taxonomically classified using the Alignment, Classification, and Tree Service (ACT) from the SILVA database [37]. The analysis showed that the standard parameters had poor resolution at higher taxonomic ranks; therefore, the best match approach was used by setting the minimal identity with query sequences to 50% and the total number of neighbors to just one. The taxonomic assignment of every group that could only be identified using standard parameters was further verified using BLAST [38] against NCBI’s database of reference RNA sequences to reduce the probability of false positive annotations. After this, all sequences classified as “Chloroplast” were removed from 16 S rRNA ASVs. Alpha rarefaction plots were constructed using the diversity plug-in in QIIME2 (Fig. S1, Supplementary material). Hierarchical clustering of the taxonomic profiles of each sample was performed using the ward method of the function cluster map from the seaborn Python package [39]. Before the hierarchical clustering, taxon abundances were normalized using the centered log-ratio transformation on each sample [40].
Cost Analysis
A preliminary cost analysis of the photosynthetic waste effluents valorization process was carried out based on the mass balance in a larger scale HRAP-ABC system, which was fed with a hypothetical biogas flow of 6 m3 h−1 with a composition of prepared mixture biogas described above, besides with the diluted organic leachate (as described above) at a hydraulic retention time of 138 d. The global results were expressed in USD sale price per biomethane cubic meter (USD m−3), where incomes and outcomes in the supplementary material section were considered for the computing. The empirical results assessed in the present work, like CO2 and H2S removal efficiencies and complete depletion of the IC supplied from the alkaline medium and biogas, were used to complete the mass balances. The supporting information section further details the investment and operational cost of the photosynthetic waste effluents valorization system.
Results and Discussion
Pilot-Scale System Performance
Figure 2 shows the TN, IC, and TSS concentrations in the HRAP for all stages of experimentation. During stage I, the superficial biomass productivity (RsX) was 5.37 g m−2 d− 1 (RX= 32.22 g m−3 d−1), lower than the values reported by Franco–Morgado et al. [30] and Marín et al. [41], who registered 4.95 g m− 2 d− 1 (55.44 g m−3 d−1) and 22.5 g m−2 d−1 (150 g m−3 d− 1) respectively, also under outdoors conditions.
At stage II, the biomass productivity lowered to 1.55 g m−2 d−1 (9.3 g m−3 d−1). Biomass productivities of 0.56 g m−2 d−1, 0.56 g m−2d−1, and 0.44 g m−2d−1 (3.36, 3.36, 2.64 g m−3 d−1) were achieved at stages III, IV, and V, respectively. These values exposed that leachate affected the growth of the microbial consortium under these culture conditions. Zhao et al. [42] reported a microalgae biomass productivity of 131 g m−3 d−1 at an optimized leachate spike ratio of 1:10 but under batch cultivations, where any nutrient does not limit initial microalgae growth.
The experimental nitrogen/biomass yield (considering only the suspended biomass) shown in Table 2 was 40% higher than the theoretical nitrogen/biomass yield, suggesting the nitrogen was used for other processes than the growth of suspended microalgae cells. One such reason could be biofilm formation and aggregates, which increased at stages II to V; however, these were not quantified. Another reason could be the NH3 stripping at basic pH. In this sense, Campos et al. [13] reported a strong influence of leachate alkalinity on ammonia nitrogen removal. They observed a direct relation between alkalinity and ammonia concentration because of prior CO2 removal, the pH increased and consequently it favored the stripping of NH3.
At stage I (batch operation mode), the TN concentration decreased, as expected, as a result of the support of the growth of microalgae biomass through nitrogen assimilation from KNO3 (Fig. 2a). From stages II to V, the TN remained in a range of 38 and 65 mg L−1, achieving the highest concentrations at stage V. In this stage, the photosynthetic system shifts to be limited by IC instead, where the lowest IC concentration and most elevated pH are attained. These conditions promoted a loss of diversity of the alkaliphilic suspended microalgae cells. They increased the suspended heterotrophic bacteria, as well as the formation of biofilm and aggregates conformed by both cyanobacteria and eukaryotic photosynthetic cells and heterotrophic bacteria.
The NH3 stripping was favored as the pH increased, explaining the higher TN concentration obtained at the lowest pH. However, the simultaneous decreasing of IC and TN concentrations at the same applied hydraulic retention time shows that NH3 from the leachate/MSM was mainly used to grow suspended cells and biofilm/aggregates. In this sense, as the leachate/MSM was fed during stages II to V, the microbial heterotrophs probably increased their proportion compared to stage I, promoting biofilm formation of both microalgae and bacteria on the available surfaces of the pilot-scale HRAP-ABC system.
The IC concentration decreased from 2200 to 1857 mg L−1 during stage I, which relates to the initial microalgae growth. However, when the mixture of leachate/MSM was fed for the first time at stage II, the IC concentration substantially increased due to the lack of control over water evaporation, which was later compensated by adding corresponding volumes of tap water in the subsequent stages. From stage III to IV, the IC concentration decreased over time until it attained 800 mg L−1 at the end of stage V when only anaerobically-produced biogas was fed (Fig. 2b). Despite this, the suspended biomass productivity during stages III to V remained stable. The decrease in the IC concentration at stages III, IV, and V is attributable to implementing a higher HRT, which reduced the IC loading rate three times compared with stage II.
Starting from stage III, the IC was also supplied as CO2 from the prepared and anaerobically-produced gas and operated under nitrogen limitation conditions for the microalgae growth. The increased heterotrophic activity likely promoted the pH drop, where the final product is CO2, increasing the absorption of acid gasses in the aqueous alkaline culture. In this sense, the CO2 transfer rates could overtake the photosynthetic activity rates, which lowered the microalgae’s capacity to produce OH− against the H+ produced during absorption.
An apparent higher IC consumption in stage V (Fig. 2b) can be explained through the IC mass balance shown in Table 2. The leachate/MSM supply was insufficient to keep a non-limiting IC substrate condition when biogas with a CO2 content of 4.4 ± 1.2%vol was fed. The growth of the photosynthetic microorganisms in this stage was limited by IC instead of nitrogen, where the lowest IC concentration and the highest pH were attained out of the whole pilot-scale system operation (Figs. 2 and 4). The consumed IC, the stripped CO2 and the weak buffer capacity of the aqueous medium reduced the carbon source bioavailability for further microalgae growth [41]. Therefore, the pH increased due to strong photosynthetic IC fixation activity and the low CO2 loading rates supplied by the anaerobically-produced biogas. Then constant monitoring is essential to establish IC supplementation for maintaining an adequate C/N for microalgae growth. The IC mass balance in the pilot-scale system for all stages indicated that CO2 stripping from the open HRAP was the primary process for promoting the elimination of CO2 from biogas. Therefore, the CO2 concentration gradient was well-maintained to allow continuous biogas upgrading. Since a significant mass proportion of CO2 was previously absorbed in the ABC, it was lost to the atmosphere as it moved towards the open HRAP, adding up to 97% of losses. This unexpected amount of CO2 stripped out from the aqueous microalgae culture was more accentuated at stages II to IV, mainly due to the low pH (around 8.5); this is caused by the instantaneous CO2 – H2O equilibrium, which enhances the CO2 stripping under pH < 7 [6].
Regarding the DO performance, Figs. 3 and 4 show the profiles for stages III, IV, and V. During diurnal periods, the highest DO concentration was around 15.5 mg L−1 , and at night, it decreased to about 1 mg L−1. However, in some daily periods, the maximal DO concentration decreased to 3 mg L−1. A DO concentration below 6.5 mg L−1 (air saturation value at Mexico City conditions) would indicate a predominant heterotrophic activity, where the population shift could explain these DO changes. This shift could mean an increase in the relative abundance of microalgal and bacterial predators (See Fig. S1 in Supplementary material), presented and discussed in the section of taxonomic analysis.
Biogas Upgrading at Different Liquid Recirculation/Biogas (L/G) Ratios
The composition of the upgraded biogas at stages III (L/G of 0.5) and IV (L/G of 1.0), when the prepared gaseous mixture was fed, is shown in Fig. 5. The results demonstrated that CH4 content was increased up to 90%vol, despite the L/G applied; however, this enhancement was not stable. The CO2 removal efficiency (CO2-RE) attained during stages III and IV was 80% ± 9. The H2S removal efficiency (H2S-RE) was almost complete, no matter the L/G ratio of the stage.
Inlet (full-square) and outlet (blank-square) composition of the biogas at L/G of 0.5 and 1.0. The prepared gaseous mixture was fed at stages III and VI. The anaerobically-produced biogas was introduced at L/G of 1.0 with an averaged composition of CH4 (53.8 ± 8.8%vol); CO2 (4.4 ± 1.2%vol); O2 (6.6 ± 2.1%vol), and N2 (36.6 ± 9.3%vol)
The DO concentration during stages III and IV ranged from 0.2 mg L−1 at nocturnal periods to 15.4 mg L−1 at diurnal periods, corroborating the existence of microalgae with photosynthetic activity. Moreover, on average, O2 concentration in the resulting upgraded biogas was 4.5 ± 2%vol. These results confirm that CH4 concentration in the upgraded biogas depends on removing CO2 and H2S and the DO stripped out in the ABC. As L/G values are higher at constant biogas inlet flow, the hydraulic residence time in the ABC is lower, and the pH is expected to be similar to the HRAP. However, the L/G did not influence the composition of the upgraded biogas regarding CO2 content, as the pH in the HRAP was around 8.5. That is contrary to what was reported in previous studies, where a CO2-RE of about 70% (L/G ratio of 0.5) and up to 90% at (L/G ratio of 1) were observed [23]. The difference can be attributed to the higher TOC concentration used in the present work, where heterotrophic activity promoted additional CO2 absorption, thus avoiding the increase of pH.
When biogas is purified by deploying a photosynthetic process, the biomethane quality is mainly limited by the O2 desorption occurring in the ABC [43]. Rodero’s research mentions that L/G ratios > 1.0 foster CO2 and H2S removals; however, the simultaneous O2 stripping would produce low-quality biomethane. In the present work, under diurnal periods, the O2 content in the upgraded biogas was quite similar for the L/G ratio of 0.5 in Stage III (5 ± 2%vol.) and L/G ratio of 1.0 in Stage IV (4 ± 2%vol.). To avoid the undesired O2 mass transference, a suitable strategy would be to decrease ABC’s DO as much as possible. Following this strategy, Franco-Morgado et al. [30] implemented an open-packed column to promote the O2 desorption to atmospheric air, reduce the HRAP effluent DO and feed this DO low-content effluent to ABC. Another suitable option would be to uptake the DO by promoting heterotrophic metabolism in the HRAP-ABC system [44] by providing an influent with soluble organic content, i.e., digestate [6].
The results obtained on the composition of the upgraded biogas after treatment complied with the technical specifications for natural gas quality, according to Mexican Legislation [45], except for the O2 content. Marín et al. [46] also reported that O2 concentration in the upgraded biogas did not comply with international regulations (≤ 1%) due to the higher DO observed in the HRAP culture broth. These results indicated that the HRAP-ABC system requires further optimization. When produced gas was fed at stage V, the H2S removal was not evaluated because this gaseous sulfur compound could not be detected at the inlet. Figure 5 shows the composition of the upgraded biogas during stage V (lasting 60 days). CH4 concentration at the outlet of the ABC increased by around 5.6%vol, achieving an average CO2-RE of 83 ± 11%. After the treatment of this anaerobically-produced gas, the maximum CH4 concentration attained was around 70%vol., which meant there was no chance to obtain biomethane, even in a CO2-RE of 100% assumed, because the influent gaseous stream composition had a poor content of both methane and carbon dioxide.
Morphological Identification of Microalgal–Bacterial Consortium
The microscopic analyses showed that the microalgal culture in the pilot HRAP-ABC system contained more than one type of microalgal cell. The dominating algal species were morphologically identified as chlorophyte (Picochlorum sp., Class Trebouxiophyceae), cyanobacteria (Pseudanabaena sp. Order Synechococcales, Spirulina sp. Order Spirulinales), and diatom (Nitzschia sp.). Qualitative screening of cell or filament counts identified the existence of microalgae in the photic zone, aphotic zone, and attached to walls. Some studies mention that strains from the genus Picochlorum are versatile microalgae for outdoor culture because this genus can grow in a wide range of salinities and temperatures [47, 48]. The most common cyanobacteria species found in significant abundance in the three zones were Pseudanabaena sp. The species Spirulina sp. was almost nonexistent in all the samples. This may be because the reactor’s pH was around 9, and the range for Spirulina growth must be between 9.5 and 10.5, with optimal biomass yield at a pH of 9.5 and a temperature of 32 °C [49]. Figure 6 shows a microscopic screening of microalgae consortium at 40X magnification, confirming high proportion of Picochlorum sp. (ovals, both in Fig. 6A and B) and Pseudoanabaena sp. (hyphae, in Fig. 6A) in the photic zone. Gao et al. [19] state that Pseudanabaena sp. is a common species in freshwater cyanobacteria blooms and conclude that water temperature and COD are the most critical factors contributing to cyanobacterial abundance. Recent studies [44, 46] identified the existence of Pseudoanabaena sp., Chlorella vulgaris, and Mychonastes homosphaera in outdoor algal-bacterial photobioreactors evaluated across different seasons of the year and deployed for biogas upgrading; these studies also observed that the dominant microalgae population in the HRAP depends on environmental parameters as ambient temperature and solar radiation. In our study, the temperature of the algal culture gradually increased due to the natural increase in ambient temperature. In the mornings, the temperature oscillated around 15 °C until it reached a maximum of 27 °C in the afternoon. During the night, it returned to the initial temperature.
Amplicon Taxonomic Analysis
The relative abundance and taxonomic classification of the ASVs are shown in Fig. S1 and S2 in supplementary materials. Taxonomic classification of 18 S rRNA assigned ASVs into four eukaryotic Superclades for the HRAP: Archaeplastida (stage I: 31%, stage IV: 93%, stage V: 53%), Discoba (stage I: 4%, stage IV: 0.03%, stage V: 0.3%), SAR (stage I: 11%, stage IV: 2%, stage V: 9%), and Amorphea (stage I: 54%, stage IV: 4%, stage V: 35%) (Fig. S1a in supplementary materials); of which only Archaeplastida and SAR encompass organisms identified as microalgae. As in the morphological analysis, the metataxonomic analysis showed the most abundant microalgae taxonomic group in the HRAP belonged to the Phylum Chlorophyta, Class Trebouxiophyceae (stage I: 27%, stage IV: 92%, stage V: 49%); it was not possible to annotate the taxonomy at the level of genus for these microalgae. Also within the Phylum Chlorophyta, three other taxonomic groups were identified, falling in the following microalgal genera: Scenedesmus (stage I: 0.3%, stage IV: 0.1%, stage V: 5%), Nephroselmis (stage I: 0, stage IV: 1%, stage V: 2%), and Chloridium (stage I: 0, stage IV: 0.06%, stage V: 3%). Additionally, in the Superclade SAR, Clade Orchrophyta, two microalgae genera were identified: Nitzschia (stage I: 3%, stage IV: 2%, stage V: 0.7%) (Phylum Bacillariophyta (Diatom)), and Chlamydomyxa (stage I: 8%, stage IV: 0.7%, stage V: 8%) (Class Chrysophyceae). It should be noted that microscopic observations also detected Nitszchia.
Other than microalgae, different 18 S rRNA ASVs were found, such as predatory microorganisms of both microalgae and bacteria. Examples present exist as protists from the genus Nuclearia (Clade Ophishtokonta) and Clyclidium (Clade Alveolata), as well as the Class Choanoflagellata (Clade Ophishtokonta). The most relevant exceptions were microorganisms classified in the genus Jakoba (Clade Discoba), bacterial-like protist heterotrophs, and two taxonomic groups in the Kingdom Fungi, organized in the Phyla Ascomycota and Chytridiomycota.
Furthermore, most 16 S rRNA ASVs were classified into five phyla: Cyanobacteria, Bacteroidota, Actinobacteria, Proteobacteria, and Verrucomicrobiota (Fig. S2a in supplementary materials). The Phyla Bacteroidota and Proteobacteria are frequently identified with microalgae in this consortia type [50]. The most abundant taxon in bacterial communities in this work was classified in the Phylum Cyanobacteria (distributed between three ASVs). However, the taxonomic classification of the dominant cyanobacteria could not be confirmed. The metataxonomic and microscopic analyses agree that filamentous cyanobacteria dominate the HRAP bacterial community. In addition, similar to morphological characterization, a low abundance of the genus Spirulina in HRAP was corroborated (0.03%, 0%, and 0.5% for stage I, stage IV, and stage V, respectively).
Hierarchical clustering of the relative abundances showed that Stage I and Stage V samples were similar (Fig. S1 and S2, upper part of Supplementary materials). A sample from Stage IV was also characterized by a significant loss of bacterial and eukaryotic diversity compared to Stage I and Stage V (Fig. S3 in supplementary materials).
Additionally, it was noticed that bacteria in the leachate had little influence on the composition of the bacterial community in the HRAP because taxonomic groups at the influent had almost no prevalence in the samples at any of the other stages (Fig. S1 in supplementary materials).
Process Cost Analysis
The economic analysis of this study was performed on the calculation base of a biogas flow of 6 m3 h−1 (generated from 1 ton of OFMSW per day), which was photosynthetically upgraded to biomethane. The economic analysis database about investment and operational cost of the photosynthetic effluents valorization process is shown in supplementary material section. The primary incomes considered here were: biomethane used for electric energy production, microalgal biomass used as soil improver (biofertilizer), and CO2 mitigation emissions certificates traded as carbon credits. In this sense, in microalgal-based technology, CO2 from biogas and nutrients are removed through biomass assimilatory mechanisms, avoiding dissipating components such as CO2 or N2O to the atmosphere and mitigating the emission of greenhouse gasses [51]. This would increase the incomes of the waste valorization process through, i.e., carbon-emission tax or carbon-emission trading, which could stimulate investment in biogas projects, mainly when these compete with fossil fuels [27]. Regarding outcomes, it mainly assumed were the energy required for the operation of the system included biogas bubbling, external cultivation broth recirculation from HRAP to the ABC, and internal recirculation of the microalgal cultivation broth in the HRAP [6].
The estimated cost to treat a biogas flow of 6 m3 h−1 resulted in USD 1.57 m−3, resulting in a convenient sale price of the obtained biomethane of USD 2.62 m−3 (considering a profit of 40%). The cost of treatment can decrease when other by-product income associated with the biological process increases in value such as microalgae biomass and carbon credits. Also, other option for decreasing the biomethane sale price could be to increase the biogas flow to be treated, in this sense, Toledo–Cervantes et al., [52] estimated treatment costs of USD 0.33 m−3 for a photosynthetically biogas upgrading plant treating a biogas flow of 300 m3 h−1. However, the sale price of biomethane is still far from being competitive with the acquisition value of natural gas for domestic service in Mexico (~ USD 0.17 m−3) [53]. In this sense, it is recommended the intervention of government funds or fiscal incentives that convert this microalgal-based organic waste valorization technology into an economically viable process.
Conclusions
Several operational strategies deployed on the microalgae valorization system induced process stability for about 400 days for biogas upgrading capacity and biomass production, where original members of the alkaliphilic microalgae consortium (i.e., Picochlorum sp. and Pseudanabaena sp.) prevailed. The nutrients that remained in organic leachate showed being bioavailable for their microalgae assimilation under the culture conditions, however the utilization of the anaerobically-produced biogas induced inorganic carbon limitation conditions for microalgae growth, due to its low content of CO2 (< 5%vol). The microalgae-based valorization system that treats a biogas flow of 6 m3 h−1 can produce biomethane, microalgae biomass for sale, in addition to carbon credits, but the price of biomethane was not competitive with natural gas, showing the need to get more incentives mainly from the respective governments.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Grand View Research: Biogas Market. Market analysis 2019. (2020). Retrieved May 12, 2023, from https://www.grandviewresearch.com/industry-analysis/biogas-market. Accessed 10 June 2023
Vochozka, M., Horák, J., Krulický, T., Pardal, P.: Predicting future brent oil price on global markets. Acta Montan. Slovaca 25, 375–392 (2020). https://doi.org/10.46544/AMS.v25i3.10
Muta, T., Erdogan, M.: The global energy crisis pushed fossil fuel consumption subsidies to an all-time high in 2022 (2023). International Energy Programme. Paris. Retrieved May 12, 2023, from https://www.iea.org/commentaries/the-global-energy-crisis-pushed-fossil-fuel-consumption-subsidies-to-an-all-time-high-in-2022
Paritosh, K., Yadav, M., Mathur, S., Balan, V., Liao, W., Pareek, N., Vivekanand, V.: Organic fraction of municipal solid waste: overview of treatment methodologies to enhance anaerobic biodegradability. Front. Energy Res 6, 75 (2018). https://doi.org/10.3389/FENRG.2018.00075/BIBTEX
Maroušek, J., Strunecký, O., Kolář, L., Vochozka, M., Kopecký, M., Maroušková, A., Batt, J., Poliak, M., Šoch, M., Bartoš, P., Klieštik, T., Filip, M., Konvalina, P., Moudrý, J., Peterka, J., Suchý, K., Zoubek, T., Cera, E.: Advances in nutrient management make it possible to accelerate biogas production and thus improve the economy of food waste processing. Energy Sour. (2020). https://doi.org/10.1080/15567036.2020.1776796
Bahr, M., Díaz, I., Dominguez, A., González Sánchez, A., Muñoz, R.: Microalgal-biotechnology as a platform for an integral biogas upgrading and nutrient removal from anaerobic effluents. Environ. Sci. Technol. 48, 573–581 (2014). https://doi.org/10.1021/ES403596M/SUPPL_FILE/ES403596M_SI_001.PDF
Awe, O.W., Zhao, Y., Nzihou, A., Minh, D.P., Lyczko, N.: A review of biogas utilisation, purification and upgrading technologies. Waste Biomass Valoriz. 8, 267–283 (2017). https://doi.org/10.1007/s12649-016-9826-4
Muñoz, R., Meier, L., Diaz, I., Jeison, D.: A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 14, 727–759 (2015). https://doi.org/10.1007/s11157-015-9379-1
Fisgativa, H., Tremier, A., Le Roux, S., Bureau, C., Dabert, P.: Understanding the anaerobic biodegradability of food waste: relationship between the typological, biochemical and microbial characteristics. J. Environ. Manag. 188, 95–107 (2017). https://doi.org/10.1016/j.jenvman.2016.11.058
Zan, F., Dai, J., Hong, Y., Wong, M., Jiang, F., Chen, G.: The characteristics of household food waste in Hong Kong and their implications for sewage quality and energy recovery. Waste Manag. 74, 63–73 (2018). https://doi.org/10.1016/J.WASMAN.2017.11.051
Grobbelaar, J.U.: Algal nutrition. In: Richmond, A. (ed.) Microalgae culture: biotechnology and applied phycology, pp. 97–115. Blackwell Publishing, UK (2004)
Gonçalves, A.L., Pires, J.C.M., Simões, M.: A review on the use of microalgal consortia for wastewater treatment. Algal Res. 24, 403–415 (2017)
Campos, J.C., Moura, D., Costa, A.P., Yokoyama, L., Araujo, F.V.D.F., Cammarota, M.C., Cardillo, L.: Evaluation of pH, alkalinity and temperature during air stripping process for ammonia removal from landfill leachate. J. Environ. Sci. Heal A 48, 1105–1113 (2013). https://doi.org/10.1080/10934529.2013.774658
Wu, K., Ho, K., Tang, C., Yau, Y.: The potential of foodwaste leachate as a phycoremediation substrate for microalgal CO2 fixation and biodiesel production. Environ. Sci. Pollut Res. 28, 40724–40734 (2021)
García, D., Alcántara, C., Blanco, S., Pérez, R., Bolado, S., Muñoz, R.: Enhanced carbon, nitrogen and phosphorus removal from domestic wastewater in a novel anoxic-aerobic photobioreactor coupled with biogas upgrading. Chem. Eng. J. 313, 424–434 (2017). https://doi.org/10.1016/j.cej.2016.12.054
Yan, C., Zhu, L., Wang, Y.: Photosynthetic CO2 uptake by microalgae for biogas upgrading and simultaneously biogas slurry decontamination by using of microalgae photobioreactor under various light wavelengths, light intensities, and photoperiods. Appl. Energy 178, 9–18 (2016). https://doi.org/10.1016/j.apenergy.2016.06.012
Arcila, J.S., Buitrón, G.: Microalgae–bacteria aggregates: effect of the hydraulic retention time on the municipal wastewater treatment, biomass settleability and methane potential. J. Chem. Technol. Biotechnol. 91, 2862–2870 (2016). https://doi.org/10.1002/jctb.4901
Franco-Morgado, M., Toledo-Cervantes, A., González-Sánchez, A., Lebrero, R., Muñoz, R.: Integral (VOCs, CO2, mercaptans and H2S) photosynthetic biogas upgrading using innovative biogas and digestate supply strategies. Chem. Eng. J. 354, 363–369 (2018). https://doi.org/10.1016/j.cej.2018.08.026
Gao, S., Hu, C., Sun, S., Xu, J., Zhao, Y., Zhang, H.: Performance of piggery wastewater treatment and biogas upgrading by three microalgal cultivation technologies under different initial COD concentration. Energy 165, 360–369 (2018). https://doi.org/10.1016/j.energy.2018.09.190
Meier, L., Barros, P., Torres, A., Vilchez, C., Jeison, D.: Photosynthetic biogas upgrading using microalgae: effect of light/dark photoperiod. Renew. Energy 106, 17–23 (2017). https://doi.org/10.1016/J.RENENE.2017.01.009
Toro-Huertas, E.I., Franco-Morgado, M., de los Cobos Vasconcelos, D., González-Sánchez, A.: Photorespiration in an outdoor alkaline open-photobioreactor used for biogas upgrading. Sci. Total Environ. 667, 613–621 (2019). https://doi.org/10.1016/j.scitotenv.2019.02.374
Franco-Morgado, M., Alcántara, C., Noyola, A., Muñoz, R., González-Sánchez, A.: A study of photosynthetic biogas upgrading based on a high rate algal pond under alkaline conditions: influence of the illumination regime. Sci. Total Environ. 592, 419–425 (2017). https://doi.org/10.1016/J.SCITOTENV.2017.03.077
Marín, D., Ortíz, A., Díez-Montero, R., Uggetti, E., García, J., Lebrero, R., Muñoz, R.: Influence of liquid-to-biogas ratio and alkalinity on the biogas upgrading performance in a demo scale algal-bacterial photobioreactor. Bioresour. Technol. 280, 112–117 (2019). https://doi.org/10.1016/j.biortech.2019.02.029
Maroušek, J., Maroušková, A., Gavurová, B., Tuček, D., Strunecký, O.: Competitive algae biodiesel depends on advances in mass algae cultivation. Bioresour. Technol. (2023). https://doi.org/10.1016/j.biortech.2023.128802
Bradley, I.M., Sevillano-Rivera, M.C., Pinto, A.J., Guest, J.S.: Impact of solids residence time on community structure and nutrient dynamics of mixed phototrophic wastewater treatment systems. Water Res. 150, 271–282 (2019)
Zhang, B., Li, W., Guo, Y., Zhang, Z., Shi, W., Cui, F., Lens, P.N.L., Tay, J.H.: Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev 118, 109563 (2020). https://doi.org/10.1016/j.rser.2019.109563
Díaz-Trujillo, L.A., Tovar-Facio, J., Nápoles-Rivera, F., Ponce-Ortega, J.M.: Effective use of carbon pricing on climate change mitigation projects: Analysis of the biogas supply chain to substitute liquefied-petroleum gas in Mexico. Processes (2019). https://doi.org/10.3390/pr7100668
Tang, C.C., Tian, Y., He, Z.W., Zuo, W., Zhang, J.: Performance and mechanism of a novel algal-bacterial symbiosis system based on sequencing batch suspended biofilm reactor treating domestic wastewater. Bioresour. Technol. 265, 422–431 (2018). https://doi.org/10.1016/j.biortech.2018.06.033
de los Cobos-Vasconcelos, D., García-Cruz, E.L., Franco-Morgado, M., González-Sánchez, A.: Short-term evaluation of the photosynthetic activity of an alkaliphilic microalgae consortium in a novel tubular closed photobioreactor. J. Appl. Phycol. (2015). https://doi.org/10.1007/s10811-015-0612-7
Franco-Morgado, M., Tabaco-Angoa, T., Ramírez-García, M.A., González-Sánchez, A.: Strategies for decreasing the O2 content in the upgraded biogas purified via microalgae-based technology. J. Environ. Manage 279, 1–7 (2021). https://doi.org/10.1016/j.jenvman.2020.111813
Campuzano, R., Trejo-Aguilar, G.M., Cuetero-Martínez, Y., Ramírez-Vives, F., Monroy, O.: Acidogenesis of food wastes at variable inlet and operational conditions. Environ. Technol. Innov. 25, 102162 (2022)
Baird, R.B., Eaton, A.D., Clesceri, L.: Standard methods for the examination of water and wastewater. American Public Health Association, Washington (2012)
Klindworth, A., Pruesse, E., Schweer, T., Peplies, J., Quast, C., Horn, M., Glöckner, F.O.: Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 41, e1–e1 (2013). https://doi.org/10.1093/nar/gks808
Hugerth, L.W., Muller, E.E.L., Hu, Y.O.O., Lebrun, L.A.M., Roume, H., Lundin, D., Wilmes, P., Andersson, A.F.: Systematic design of 18S rRNA gene primers for determining eukaryotic diversity in microbial consortia. PLoS ONE 9, e95567 (2014)
Bolyen, E., Rideout, J.R., Dillon, M.R., Bokulich, N.A., Abnet, C.C., Al-Ghalith, G.A., et al.: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. (2019). https://doi.org/10.1038/s41587-019-0209-9
Callahan, B.J., McMurdie, P.J., Rosen, M.J., Han, A.W., Johnson, A.J.A., Holmes, S.P.: DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016). https://doi.org/10.1038/nmeth.3869
Pruesse, E., Peplies, J., Glöckner, F.O.: SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics 28, 1823–1829 (2012). https://doi.org/10.1093/bioinformatics/bts252
Altschul, S.F., Gish, W., Miller, W., Myers, E.W., Lipman, D.J.: Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). https://doi.org/10.1016/S0022-2836(05)80360-2
Waskom, M.: Seaborn: statistical data visualization. J. Open. Source Softw. 6, 3021 (2021). https://doi.org/10.21105/joss.03021
Gloor, G.B., Macklaim, J.M., Pawlowsky-Glahn, V., Egozcue, J.J.: Microbiome datasets are compositional: and this is not optional. Front. Microbiol. 8, 1–6 (2017). https://doi.org/10.3389/fmicb.2017.02224
Marín, D., Posadas, E., Cano, P., Pérez, V., Blanco, S., Lebrero, R., Muñoz, R.: Seasonal variation of biogas upgrading coupled with digestate treatment in an outdoors pilot scale algal-bacterial photobioreactor. Bioresour. Technol. 263, 58–66 (2018). https://doi.org/10.1016/j.biortech.2018.04.117
Zhao, X., Zhou, Y., Huang, S., Qiu, D., Schideman, L., Chai, X., Zhao, Y.: Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour. Technol. 156, 322–328 (2014). https://doi.org/10.1016/j.biortech.2013.12.112
Rodero, M.R., Lebrero, R., Serrano, E., Lara, E., Arbib, Z., García-Encina, P.A., Muñoz, R.: Technology validation of photosynthetic biogas upgrading in a semi-industrial scale algal-bacterial photobioreactor. Bioresour. Technol. 279, 43–49 (2019). https://doi.org/10.1016/j.biortech.2019.01.110
Cantera, S., Fischer, P.Q., Sánchez-Andrea, I., Marín, D., Sousa, D.Z., Muñoz, R.: Impact of the algal-bacterial community structure, physio-types and biological and environmental interactions on the performance of a high rate algal pond treating biogas and wastewater. Fuel (2021). https://doi.org/10.1016/j.fuel.2021.121148
Diario Oficial de la Federación: NOM-001-SECRE-2010, Especificaciones del gas natural. Última reforma publicada DOF 19-03-2010. 18–35 (2010). Retrieved May 5, 2023, from https://www.dof.gob.mx/normasOficiales/3997/sener/sener.htm. Accessed 10 June 2023
Marín, D., Méndez, L., Suero, I., Díaz, I., Blanco, S., Fdz-Polanco, M., Muñoz, R.: Anaerobic digestion of food waste coupled with biogas upgrading in an outdoors algal-bacterial photobioreactor at pilot scale. Fuel (2022). https://doi.org/10.1016/j.fuel.2022.124554
De la Vega, M., Díaz, E., Vila, M., León, R.: Isolation of a new strain of Picochlorum sp and characterization of its potential biotechnological applications. Biotechnol. Prog. 27, 1535–1543 (2011)
Krasovec, M., Vancaester, E., Rombauts, S., Bucchini, F., Yau, S., Hemon, C., Lebredonchel, H., Grimsley, N., Moreau, H., Sanchez-Brosseau, S., Vandepoele, K., Piganeau, G.: Genome analyses of the microalga Picochlorum provide insights into the evolution of thermotolerance in the green lineage. Genome Biol. Evol 10, 2347 (2018). https://doi.org/10.1093/GBE/EVY167
Soni, R.A., Sudhakar, K., Rana, R.S.: Comparative study on the growth performance of Spirulinaplatensis on modifying culture media. Energy Rep. 5, 327–336 (2019). https://doi.org/10.1016/J.EGYR.2019.02.009
Cho, H.U., Kim, Y.M., Choi, Y.N., Kim, H.G., Park, J.M.: Influence of temperature on volatile fatty acid production and microbial community structure during anaerobic fermentation of microalgae. Bioresour. Technol. 191, 475–480 (2015). https://doi.org/10.1016/j.biortech.2015.03.009
Nagarajan, D., Lee, D.J., Kondo, A., Chang, J.S.: Recent insights into biohydrogen production by microalgae – from biophotolysis to dark fermentation. Bioresour. Technol. 227, 373–387 (2017). https://doi.org/10.1016/j.biortech.2016.12.104
Toledo-Cervantes, A., Estrada, J.M., Lebrero, R., Muñoz, R.: A comparative analyses of biogas upgrading technologies: photosynthetic vs physical/chemical processes. Algal Res. 25, 237–243 (2017)
Natural Gas Price Reference Indices. Mexico. https://www.cre.gob.mx/IPGN/. Accessed 8 May 2023
Acknowledgements
The technical support of Miguel Angel Ramirez Garcia, Rosalinda Campuzano, Margarita Elizabeth Cisneros Ortiz, Roberto Sotero Briones Méndez, Arnold Ramirez Rueda and Wenceslao Bonilla Blancas is highly appreciated. This research was done at IIUNAM-Environmental Engineering Laboratory with an ISO 9001:2015 certificate.
Funding
This research was supported by the Mexican Secretary of Energy and the National Science Foundation of Mexico (Fondo Sectorial CONACyT-SENER, CEMIE-Bio ID project 247006, Mexico). We thank DGAPA UNAM project number IT100423 for the partial funding.
Author information
Authors and Affiliations
Contributions
AV: formal analysis, data curation, writing–original draft. MFM: formal analysis, investigation, review and editing. AS: data curation, investigation. YCM: investigation, data curation, review and editing. GB: supervision, project administration, review and editing. DCV: supervision, review and editing. OM: conceptualization, writing–review. AGS: conceptualization, methodology, supervision, writing–review and editing, project administration. The manuscript has been read and approved for submission by all the named authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velasco, A., Franco-Morgado, M., Saldivar, A. et al. Organic Leachate and Biogas Utilization in Outdoor Microalgae Cultivation Under Alkaline Conditions at Pilot-Scale. Waste Biomass Valor 15, 4265–4277 (2024). https://doi.org/10.1007/s12649-023-02223-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02223-3