Abstract
The main goal of this study was to investigate in vitro the antibacterial action of chitosan (CS), in various physical forms (film, bead, powder and solution), against selected bacteria including Escherichia coli, Staphylococcus epidermidis, Proteus mirabilis and Pseudomonas aeruginosa. For this purpose, chitosan was produced by N-deacetylation of chitin under different alkaline conditions. Degree of deacetylation (DDA), molecular weight and viscosity of each form of chitosan were determined. The functional groups of chitosan were confirmed by Fourier Transform Infrared Spectroscopy and the degradation temperatures by Differential Scanning Calorimetry (DSC). Chitosan with high DDA (95%), molecular weight (149.01 KDa) and viscosity (0.2498 dL/g) was obtained by deacetylation with 50% of sodium hydroxide at 90 °C for 4 h. The antibacterial test results demonstrated the presence of significant antibacterial action of soluble chitosan against the tested pathogenic bacteria. In addition, the bacterial growth showed a considerable decrease in bacterial biomass after addition of CS, the resistance of the studied bacterial strains against soluble CS decreased in the following order: E. coli > P. mirabilis > S. epidermidis and their action process was bacteriostatic rather than bactericidal.
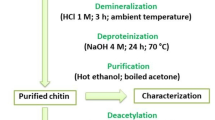
Graphical Abstract








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this paper and its supplementary information files.
References
Hirano, S.: Production and application of chitin and chitosan in Japan. In: Skjak-Braek, G., Anthonsen, T., Sanford, P. (eds.) Chitin and chitosan: sources, chemistry, biochemistry, physical properties and applications, pp. 37–43. Elsevier Science Publishers, Essex (1989)
Webster, C., Onokpise, O., Abazinge, M., Muchovej, J., Johnson, E., Louime, C.: Turning waste into usable products: a case study of extracting chitosan from blue crab. Am. J. Environ. Sci. 10(4), 357–362 (2014)
Triunfo, M., Tafi, E., Guarnieri, A., Scieuzo, C., Hahn, T., Zibek, S., Salvia, R., Falabella, P.: Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals. Cosmetics 8(2), 40 (2021)
Kumari, S., Rath, P.K.: Extraction and characterization of chitin and chitosan from (Labeo rohit) fish scales. Procedia Mater. Sci 6, 482–489 (2014)
Muzzarelli, R.A.A., Muzzarelli, C.: Chitin and chitosan hydrogels. In: Phillips, G.O., Williams, P.A.(eds), Handbook of Hydrocolloids (Second Edition), pp. 849–888. Woodhead Publishing, Cambridge (2009). https://doi.org/10.1533/9781845695873.849
Ssekatawa, K., Byarugaba, D.K., Wampande, E.M., Moja, T.N., Nxumalo, E., Maaza, M., Sackey, J., Ejobi, F., Kirabira, J.B.: Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 11, 4116 (2021)
Triunfo, M., Tafi, E., Guarnieri, A., Salvia, R., Scieuzo, C., Hahn, T., Zibek, S., Gagliardini, A., Panariello, L., Coltelli, M.B., De Bonis, A., Falabella, P.: Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci. Rep. 12(1), 6613 (2022)
Ke, C.-L., Deng, F.-S., Chuang, C.-Y., Lin, C.-H.: Antimicrobial actions and applications of chitosan. Polymers 13(6), 904 (2021)
Raja, P., Chellaram, C., John, A.A.: Antibacterial properties of chitin from shell wastes. Indian J. Innov. Dev. 1, 7–11 (2012)
Friedman, M., Juneja, V.K.: Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Protect 73, 1737–1761 (2010)
Kong, M., Chen, X.G., Xing, K., Park, H.J.: Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 144, 51–63 (2010)
Confederat, L.G., Tuchilus, C.G., Dragan, M., Sha’at, M., Dragostin, O.M.: Preparation and antimicrobial activity of chitosan and its derivatives: a concise review. Molecules 26, 3694 (2021)
Goy, R.C., Morais, S.T.B., Assis, O.B.G.: Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Revista Brasileira de Farmacognosia 26(1), 122–127 (2016)
Shi, B., Zhang, H., Shen, Z., Bi, J., Dai, S.: Developing a chitosan supported imidazole schiff-base for high-efficiency gene delivery. Polym. Chem. 4, 840–850 (2013)
Devlieghere, F., Vermeulen, A., Debevere, J.: Chitosan: antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 21, 703–714 (2004)
Helander, M., Nurmiaho-Lassila, E.L., Ahvenainen, R., Rhoades, J., Roller, S.: Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 71(2–3), 235–244 (2001)
Duan, C., Meng, X., Meng, J., Khan Md, I.H., Dai, L., Khan, A., An, X., Zhang, J., Huq, T., Ni, Y.: Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 4(1), 11–21 (2019)
Guarnieri, A., Triunfo, M., Scieuzo, C., Ianniciello, D., Tafi, E., Hahn, T., Zibek, S., Salvia, R., De Bonis, A., Falabella, P.: Antimicrobial properties of chitosan from different developmental stages of the bioconverter insect hermetia illucens. Sci. Rep. 12, 8084 (2022)
Ardean, C., Davidescu, C.M., Nemeş, N.S., Negrea, A., Ciopec, M., Duteanu, N., Negrea, P., Duda-Seiman, D., Musta, V.: Factors influencing the antibacterial activity of chitosan and chitosan modified by functionalization. Int. J. Mol. Sci. 22, 7449 (2021). https://doi.org/10.3390/ijms22147449
Ramachandiran, S., Satpaty, S., Shankar, K., Kalimuthu, K., Muthuvel, A.: Anti-cancer properties of protein hydrolysate from the posterior salivary gland of amphioctopus membranaceus (Quoy & Gaimard, 1832). Int. J. Pept. Res. Ther. 26(3), 1429–1436 (2020)
Liu, N., Chen, X.G., Park, H.J., Liu, C.G., Liu, C.S., Meng, X.H.: Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 64(1), 60–65 (2006)
Ramasamy, P., Barwin Vino, A., Saravanan, R., Subhapradha, N., Shanmugam, V., Shanmugam, A.: Screening of antimicrobial potential of polysaccharide from cuttlebone and methanolic extract from body tissue of sepia prashadi Winkworth, 1936. Asian Pac. J. Trop. Biomed. 1(2), S244–S248 (2011)
Boudouaia, N., Bengharez, Z., Jellali, S.: Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: application for eriochrome black T removal from aqueous solutions. Appl. Water Sci. 9(4), 1–12 (2019)
Mokeddem, A., Benykhlef, S., Bendaoudi, A.A., Boudouaia, N., Mahmoudi, H., Bengharez, Z., Topel, S.D., Topel, Ã.: Sodium alginate-based composite films for effective removal of congo red and Coralene dark red 2B dyes: kinetic, isotherm and thermodynamic analysis. Water 15, 1709 (2023)
Qu, B., Luo, Y.: Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors– A review. International Journal of Biological Macromolecules 152, 437–448 (2020)
Bauer, A.W., Kirby, W.M., Sherris, J.C., Turck, M.: Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45(4), 493–496 (1966)
Henry M, Edy AM, Desmarest P, Manoir JD: Glycyrrhiza glabra L (Licorice): cell culture, regeneration, and the production of glycyrrhizin. In: Bajaj YPS (eds) Medicinal and aromatic plants III biotechnology in agriculture and forestry. Vol. 15. Springer, Heidelberg (1991)
Tokatlı, K., Demirdoven, A.: Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Process. Preserv. (2017). https://doi.org/10.1111/jfpp.13494
Sreerag, G., Sabu, T., Anitha, P.: Handbook of chitin and chitosan, 1st edn. Elsevier, Amsterdam (2020). ISBN 9780128179703
Wang, X.K., Wang, J.G., Guo, P.Q., Guo, W.L., Li, G.L.: Chemical effect of swirling jet-induced cavitation: degradation of rhodamine B in aqueous solution. Ultrason. Sonochem. 15, 357–363 (2008)
Chen, R., Wang, X., Yao, X., Zheng, X., Wang, J., Jiang, X.: Near-IR-triggered photothermal/photodynamic dual-moda lity therapy system via chitosan hybrid nanospheres. Biomaterials 34(33), 8314–8322 (2013)
Muzzarelli, R.A.A., Tanfani, F., Emanuelli, M., Gentile, S.: The chelation of cupric ions by chitosan membranes [Callinectessapidus, blue crab shell]. Int. J. Appl. Biotechnol. Biochem. 2, 380–389 (1980)
Ahlafi, H., Moussout, H., Boukhlifi, F., Echetna, M., Bennani, M.N., Slimane, S.M.: Kinetics of N-deacetylation of chitin extracted from shrimp shells collected from coastal area of Morocco. Mediterranean J. Chem. 2(3), 503–513 (2013)
Muzzarelli, R.A.A., Rocchetti, R.: Determination of the degree of acetylation of chitosans by first derivative ultraviolet spectrophotometry. Carbohydr. Polym. 5(6), 461–472 (1985)
No, H.K., Hur, E.Y.: Control of foam formation by antifoam during demineralization of crustacean shell in preparation of chitin. J. Agric. Food Chem. 46, 3844–3846 (1998)
Miya, M., Iwamoto, R., Mima, S.: FTIR study of intermolecular interactions in polymer blends. J. Polym. Sci. 22, 1149–1151 (1984)
Li, B., Wu, X., Bao, B., Guo, R., Wu, W.: Evaluation of α-chitosan from crab shell and β-chitosan from squid gladius based on biochemistry performance. Appl. Sci. 11, 3183 (2021)
Domard, A., Domard, M.: Chitosan: structure-properties relationship and biomedical applications. In: Dumitriu, S. (ed.) Polymeric Biomaterials, 2nd edn., pp. 187–212. Marcel Dekker Inc, New York (2002)
Moller, H., Grelier, S., Pardon, P., Coma, V.: Antimicrobial and physico–chemical properties of chitosan-HPMC-based films. J. Agric. Food Chem. 54, 3290–3296 (2004)
Ponce, A.G., Fritz, R., Del valle, C., Roura, S.I.: Antimicrobial activity of essential oils on the native microflora of organic swiss chard. LWT Food Sci. Technol. 36(7), 679–684 (2003)
Chen, C.S., Liau, W.Y., Tsai, G.J.: Antibacterial effects of N-sulfonated and N-sulfobenzoyl chitosan and application to oyster preservation. J. Food Prot. 61, 1124–1128 (1998)
Liu, L., Li, S., Garreau, H., Vert, M.: Selective enzymatic degradations of poly (L-lactide) and poly (ε-caprolactone) blend films. Biomacromol 1, 350–359 (2000)
Arkoun, M., Daigle, F., Heuzey, M.-C., Ajji, A.: Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules. 22(4), 585 (2017)
Benhabiles, M.S., Salah, R., Lounici, H., Drouichhe, N., Goosen, M.F.A., Mameri, N.: Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 29, 48–56 (2012)
Rabea, E.I., Badawy, M.E.T., Evens, C.V., Smagghe, G., Steurbaut, W.: Chitosan as antimicrobial agent: applications and mode of action. Biomacromol 4, 1457–1465 (2003)
Xie, Y.J., Liu, X.F., Chen, Q.: Synthesis and characterization of water-soluble chitosan derivate and its antibacterial activity. Carbohydr. Polym. 69, 142–147 (2007)
Malinowska-Pañczyk, E., Wojtasz-Pajk, A., Staroszczyk, H., Gottfried, K., Koodziejska, I.: Antimicrobial properties of chitosan solutions, chitosan films and gelatin-chitosan films. Polimery 60, 11–12 (2015)
Chung, Y.C., Su, Y.P., Chen, C.C., Jia, G., Wang, H.I., WU, J.C.G., Lin, J.G.: Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 25(7), 932 (2004)
No, H.K., Park, N.Y., Lee, S.H., Meyers, S.R.: Antibacterial activity of chitosans and oligomers with different molecular weights. Int. J. Food Microbiol. 74, 65–72 (2002)
Divya, K., Vijayan, S., Tijith, K.G., Jisha, M.S.: Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym. 18, 221–230 (2017)
Vilar-Junior, J.C., Ribeaux, D.R., Alves da Silva, C.A., Campos Takaki, D., Maria, G.: Physicochemical and antibacterial properties of chitosan extracted from waste shrimp shells. Int. J. Microbiol. (2016). https://doi.org/10.1155/2016/5127515
Abbouni, B., Elhariry, H.M., Auling, G.: Arrest of cell cycle by inhibition of ribonucleotide reductase induces accumulation of NAD+ by Mn2+-supplemented growth of Corynebacterium ammoniagenes. Biotechnol. Lett. 25(2), 143–147 (2003)
Jing, Y., Hao, Y., Qu, H., Shan, Y., Li, D., Du, R.: Studies on the antibacterial activities and mechanisms of chitosan obtained from cuticles of housefly larvae. Acta Biol. Hung. 58, 75–86 (2007)
Lou, M.M., Zhu, B., Muhammad, I., Li, B., Xie, G.L., Wang, Y.L., Li, H.Y., Sun, G.C.: Antibacterial activity and mechanism of action of chitosan solutions against apricot fruit rot pathogen Burkholderia seminalis. Carbohydr. Res. 346, 1294–1301 (2011)
Raafat, D., von Bargen, K., Haas, A.: Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 74, 3764–3773 (2008)
Silva, L.P., Britto, D., Seleghim, M.H.R., Assis, O.B.G.: In vitro activity of water-soluble quaternary chitosan chloride salt against Ecoli. World J. Microbiol. Biotechnol. 26, 2089–2092 (2010)
Eaton, P., Fernandes, J.C., Pereira, E., Pintado, M.E., Malcata, F.X.: Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 108, 1128–1134 (2008)
Acknowledgements
The authors would like to thank the Directorate General of Scientific Research and Technological Development (DGRSDT) and the Ministry of Higher Education and Scientific Research (MESRS), Algeria, for scientific support. Also, we are very thankful to the staff of the central laboratory of the Hospital of Sidi Bel Abbes city who facilitated the antibacterial tests and contributed to the successful of this study.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that there is no conflict of interest regarding this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boudouaia, N., Benine, M.L., Fettal, N. et al. Antibacterial Action of Chitosan Produced from Shrimp Waste Against the Growth of Escherichia coli, Staphylococcus epidermidis, Proteus mirabilis and Pseudomonas aeruginosa. Waste Biomass Valor 15, 1267–1279 (2024). https://doi.org/10.1007/s12649-023-02220-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-023-02220-6