Abstract
Purpose
Coffee silverskin (CS) is the integument covering the raw coffee bean, representing the primary waste product of the coffee-roasting industry. Despite the growing attention in seeking potential reuse of this material, the majority of CS is commonly used as a firelighter or discharged to landfills. The study aimed to test co-composting as a low-cost solution that meets the circular economy paradigms proposed by the European Union.
Methods
Four composting mixtures were prepared mixing CS with pruning waste and biochar at different ratios, aiming to maximize the amount of compostable CS per batch and monitored for 60 days.
Results
The contents of macro-, micro- and trace elements of the final composts matched the strictest requirements of the Spanish national regulation on compost quality (Class A amendments), proving that CS composts area high-value amendment rich in N and K.
Despite the highly phytotoxic effect of CS raw material, the seed germination tests showed that all the mature composts exhibited phytostimulant properties allowing their harmless application to the soil. The four composts had a high water holding capacity (237–351% dw) and they are likely to promote the persistence of plant-available water in the soil.
Conclusion
The present study showed that composting the whole CS produced in Europe would lead to a recovery of 2420–3481 tons of nitrogen and 1873 tons of potassium, reducing the dependency on mineral fertilizers, thus meeting the growing demand for sustainable and low-cost amendments.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of novelty
Coffee silverskin is the protective layer of the coffee beans, the main by-product of coffee roasting process that largely accumulates in consuming countries. Despite its high content in nitrogen and potassium, and its extremely high water retention capacity, the presence of phytotoxic compounds limited the use of coffee silverskin as soil amendment. In this study we demonstrate that co-composting with carbon rich materials, such as gardening prune and biochar, is a valid alternative to remove the phytotoxicity effect of raw coffee silverskin maintaining its beneficious agronomical properties. Composting coffee silverskin appears to be an easy process and a low-cost opportunity: up to 3481 tons of N and 1873 tons of K could be recovered in Europe from this by-product.
Introduction
The agro-food industries produce 1.3 billion tons of wastes and by-products, which accounts for one trillion USD of economic losses [1]. Alternative processing of this organic waste fraction would help to reach “recycling, recovering, and reusing” models, overcoming the linear “take-make-dispose” one [2]. Nowadays, EU is promoting research on sustainable practices to recover high-quality resources from organic residues and by-products with the Green Deal objectives and the new Circular Economy Action Plan [3].
Coffee is one of the most consumed beverages worldwide. In particular, the global coffee consumption in 2019/2020 reached 9.84 million tons, and Europe was the largest consumer with 3.35 million tons [4]. Coffee production and consumption generate large amounts of by-products like immature and defective beans, coffee husks, pulp, coffee silverskin (CS), and spent coffee grounds. Among all of these by-products, CS and spent coffee grounds account for 50% of the total waste produced by the coffee industry. However, in consuming countries, these residues are the only by-products produced locally, either during the roasting process or the final consumption of the beverage [5]. Coffee silverskin is an integument covering the raw coffee bean [6] that represents 4.2% of coffee green beans' total weight [7, 8]; this implies that Europe had to treat 166,670 tons of this by-product in 2019/2020 [9]. Coffee silverskin is commonly used as firelighter or discharged to landfills, even if alternative uses as raw material for functional aliments [10, 11], bio-oils [12] and cosmetic products [13] have also been proposed. However, the processes to obtain these products with high added value leads to not often satisfactory yields, moreover they require technical expertise, several extraction and treatment methods and a costly infrastructure, and this can hamper the viability at a large scale [12, 13].
Composting embraces circular economy and the new paradigm of the industrial model: (i) generating added value by-products (primarily environmental value), (ii) reducing greenhouse gas emissions, (iii) creating new employments [3]. Composting is a low-cost solution to agro-industrial waste, which creates soil amendments that improve nutrient availability, promote C sequestration, and enhance soil biological quality [14]. The high macro and micro-nutrient contents—e. g. potassium (21.1 g kg−1 of dry CS), calcium (9.4 g kg−1), magnesium (3.1 g kg−1), sulphur (2.8 g kg−1), and iron (0.843 g kg−1)—make CS an attractive resource as horticultural fertilizer [5, 8]. González-Moreno et al. suggested that coffee industrial wastes can undergo bio-oxidative processes, obtaining a stabilized composted material that can be used as a soil amendment [15], while Ronga et al. stated CS could replace partially peat and synthetic fertilizers for potted plants [16]. However, composting CS is not always a straightforward process, since the raw material presents plant phytotoxicity due to the high content of polyphenols and chlorogenic acid [17]. Moreover, the low bulk density and high water-holding capacity (WHC) of CS can cause pile compaction, entailing the risk of anaerobiosis, hence odours and N losses. Finally, the acidic pH and the salinity of CS should be considered as drawbacks in composting processes. To overcome these problems, CS should be mixed with co-composting materials like garden pruning waste or biochar with higher C/N ratio and better bulking properties that allow airflow to enhance aerobic digestion.
This work aimed to investigate the potential of CS to be co-composted with pruning waste and biochar to obtain a stabilized, nutrient-rich soil amendment constituting a valid alternative to mineral fertilizers. For this purpose, the composting process of four mixtures of CS, pruning waste and biochar at different proportions was monitored, focusing on the evolution of temperature, moisture, carbon (C) and nitrogen (N) forms of the composted materials. To establish the agronomic value a complete characterization of the obtained final composts was performed, including germination assays.
Material and Methods
Raw Materials
Coffee silverskin was provided by Café Candelas SA (Lugo, Spain). This by-product originated from a coffee roasting process carried out with hot air ventilation using a temperature ramp up to 210 °C for 12 min to separate CS from coffee beans. After separation, CS was humidified and pressed in sealed plastic bags to ease storage and disposal. Due to the high roasting temperature before the pressing, CS undergone a “sterilization-like” process and it was not affected by mould proliferation inside the storage bags. Gardening pruning waste (GP) was provided by a local pruning company based in Brunete (Madrid, Spain) and consisted of a mix of tree trunks, branches and leaves of different species (mainly poplars and oaks). Biochar (BC) was provided by the Carbón Vivo SCCL (Barcelona, Spain), produced from Aleppo pine woods trunks and branches, pyrolyzed in a Kon Tiki Kiln oven at 650–750 °C for 3.5 h. The main physical and chemical characteristics of the three raw materials are reported in Table 1.
Composting Process and Sampling
To maximize the used proportion of CS and to achieve optimum composting parameters and the highest quantity of nutrient in the final composts, four co-composting mixtures of the raw materials were prepared as explained in Table 2.
The mixtures were composted into expanded polypropylene composters with a volume capacity of 200 L and a passive aeration system (HOTBIN composting, Northampton, UK). Gardening prune was moistened before mixing to optimal initial values (~ 60%); moisture was maintained at 40–60% during the whole composting process by watering the mixtures with a sprayer. The mixtures were left to stand during the three initial days, and subsequently stirred manually every 3 days to ensure aeration and homogenization.
Composite samples representing each pile were collected at the beginning (day 0) and after 3, 9, 20, and 30 days of composting. The samples were immediately stored at 4 °C before analyses. Temperature within the piles was continuously measured using probes installed at 30 cm depth in the mixtures and a measuring unit (Decagon Datalogger).
After 30 days of composting, the mixtures were removed and left in the open air for 30 more days to reach adequate maturity for agronomic purposes.
Physical, Chemical and Phytotoxicity Analyses
Moisture content of the collected samples was measured using a Radwag MA 110.R moisture balance equipped with infrared lamps. The pH and electrical conductivity (EC) were measured in water-compost extracts 1:10 (w/v) using a pH meter (CRISON micropH 2001) and a conductivity meter (CRISON microCM 2201), respectively. The total carbon (C) and nitrogen (N) contents were determined by dry combustion using a Thermo Flash 2000 NC Soil Analyser. Ammonium was extracted in KCl 1 M (1:10 w/v) and determined using a visible spectrophotometer HACH Lange DR2800 with pre-dosed testing cuvettes HACH LCK 303 and LCK 304 depending on the concentration range. Nitrate was extracted in water (1:10 w/v) and measured with the same spectrophotometer using HACH LCK 339 pre-dosed testing cuvettes. The contents of nutrient and trace elements were determined after nitric (HNO3) and perchloric acid (HClO4) digestion in a sand bath at 200 °C with an induction coupled plasma atomic emission spectrophotometer (ICP-AES, Perkin Elmer Optima 4300 DV). All the analyses were performed in triplicate, except for macro, micronutrients and trace elements, for which a composite sample per treatment was analysed.
EC, pH, ammonium and nitrate were analysed on fresh samples, while C, N, macro, micronutrients and heavy metals were analysed on air-dried samples finely ground with a ball mill (RETSCH MM400).
To determine the water holding capacity (WHC), samples of the raw materials and the final composts were placed in disposable plastic cup and oven-dried at 40 °C to calculate their dry weight. The samples were brought to saturation with water for 8 h. Afterward, the plastic cups were covered at the top with parafilm and then perforated at the bottom to allow the leaching of the excess water for 24 h. Later on, the material was reweighed to measure the difference between the dry and the wet material so the amount of water the material could retain.
The phytotoxicity of the matured composts and raw materials was evaluated by seed germination tests conducted in triplicate. Ten seeds of garden cress (Lepidium sativum) were placed in Petri dishes in which five millilitres of aqueous extract of each material were added (1/10 w/v). Controls consisted of deionized water. Seed germination rate (G) and root length (L) were measured after a period of 72 h of incubation at 25 °C in darkness. The germination index (GI) was calculated as:
where G and L refer to seed germination rate and average root length, respectively, and the subscript “extract” and “control” refers to the values obtained for the extract solution from the materials and the controls with water, respectively [18].
Statistical Analysis
Analysis of variance (ANOVA) was used to examine the differences among the physical, chemical and phytotoxicity properties of the composts. When the P-value for the ANOVA was < 0.05, means were compared using a Tukey's test at the 0.05 level. All statistical analyses were conducted using R version 4.1.1 (R Core Team, 2021) using the packages: tidyverse, readxl, lme4, lmerTest, multcomp, multcompView, emmeans, clipr, viridis, patchwork, dplyr, forcats, tibble, ggplot2, and svglite.
Results and Discussion
Stages of Composting: Trends for pH and Electrical Conductivity
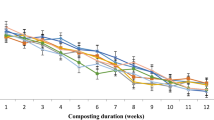
The mixtures underwent a first mesophilic phase (18–45 °C), a thermophilic phase (45–70 °C), and a second mesophilic stage, followed by the maturation phase, which took place in the open air for thirty days (Fig. 1a). The first mesophilic phase was longer for mixture 4 compared to the other mixtures. The temperature of mixture 1 dropped after the second addition of CS but increased again three days after, reaching 59.7 °C five days after.
The temperature increases are normally attributed to the metabolization of easily soluble compounds (e.g., sugars, starch, and simple proteins) and the insulation provided by the composting mass against heat loss [19]. The thermophilic phase lasted 25 days for mixture 1, 24 days for mixture 2, 6 days for mixture 3, and 13 days for mixture 4; the respective peak temperature recorded were of 59.7, 63.4, 56.9, and 58.5 °C, respectively. An exposure time of at least two weeks at 55 °C is considered the optimum condition to effectively eliminate human and plant pathogens, along with weed seeds and insect larvae [19]. The shorter thermophilic phase of mixture 3 may be attributed to the heap compaction registered during the composting process [19]. For mixture 2, the rapid increase in temperature and the overall temperature profile could attributed to the BC presence. Biochar may fill free spaces between particles of raw materials and reduce heat losses during composting [20]. Moreover, BC addition provides suitable conditions for the proliferation and activity of microorganisms through increased aeration, water and nutrient retention, and toxin adsorption [21]. Temperature drops registered after around 30 days indicated the slowdown of microbiological processes, and that all the mixtures reached stability, even before the maturation phase that lasted other 30 days.
Moisture content of the four mixtures was maintained between 40 and 60% during the whole process to assure the optimal conditions for the microbial communities (Fig. 1b). The moisture content for mixture 4 was purposely adjusted to a lower value to avoid the compaction and the emission of ammonia, due to the high content of CS. On the contrary, the coarser texture of mixture 1 led to larger water evaporation during the first ten days.
At the beginning of composting, mixtures 1, 2, and 3 had a slightly alkaline pH (pH 7–8), while mixture 4 showed an acidic pH (Fig. 2a) due to the higher content of CS, which had a pH of 5.5 (Fig. 2a). During composting, the pH of all the mixtures increased to 8 and 9 during the first week, and then became constant after 30 days except for mixture 1, where a slight decrease in pH was observed during the maturation process. At the end of composting, the pH of mixture 1 was slightly lower than those of mixtures 2 and 3 (8.5) and especially that of mixture 4 (9) (Fig. 2a). The increase in pH throughout the composting process may be attributed to the release of ammonia associated with protein degradation, while the subsequent decrease during the maturation stage may be caused by the volatilization of ammoniacal nitrogen and proton release resulting from the microbial nitrification process [22]. The pH of the mixtures is relevant for controlling N-losses by ammonia volatilisation, which may be particularly high if pH raises above 7.5. Furthermore, pH values of 6.7–9.0 support good microbial activity during composting and pH levels lower than 9 indicated that the four mixtures were safe for plant health and agricultural use [23].
The electrical conductivity of the mixture increased with composting time, especially that of mixtures 1, 3, and 4, which had values above 2 dS m−1 at the end of composting (Fig. 2b). As organic matter degrades, the concentration of cations increases with a surge of EC, so the salinity of the material [19]. The lower EC of mixture 2 (below 2 dS m−1) might be attributed to the presence of reactive surfaces within the high pore space of biochar, which may enhance adsorption of cations. This adsorption mechanism is normally described as a results of the interactions of cations with the functional groups in biochar skeleton [24] or with the π electrons of the condensed aromatic rings, or the formation of colloids [25].
High salinity may cause osmotic stress, which may negatively affect microbial populations involved in the composting process and limit plant growth and productivity. Recommended limits of electric conductivity are around 2 dS m−1, although Solanaceae (tomatoes, aubergines, etc.) tolerate values up to 2.5 dS m−1 [26], and other crops such as barley, cotton, and wheat can tolerate levels as high as 8 dS m−1 [27].
Overall, all the final composts reached satisfactory values of pH and EC. The presence of biochar seems to improve the thermophilic profile and reduce the electrical conductivity of the mixtures, but barely affect their pH.
Carbon and Nitrogen Dynamics During Composting
The carbon content of mixtures 1, 3, and 4 slightly decreased throughout the composting process, a common trait of organic matter degradation (Fig. 3a). However, mixture 2 had an opposite trend due to the addition of BC. At the end of composting, the organic C contents of mixtures 1, 3, and 4 were significantly lower than that of mixture 2 (Fig. 3a). The increase of C content of mixture 2 can be attributed to the high stability of biochar C, which is likely to be left barely intact during the process, and the relative higher loss of other elements with composting. Indeed, its structure gives biochar higher stability against microbial degradation carbon, meaning that carbon pools from biochar have longer persistence times and a higher carbon sequestration potential than labile carbon sources from silverskin and pruning waste [28]. In addition, the presence of functional groups on the biochar surface leads to the chemisorption of the dissolved organic carbon [21] rendering them stable and contributing to the increasing trend for C content observed for mixture 2. These results highlight the positive effects of BC a co-composting agent to reduce the amount of organic fraction degraded and GHG emissions [29].
The nitrogen content of the mixtures without biochar tended to increase during the composting, while that of the mixture with BC remained constant (Fig. 3b). Final compost from mixture 2 had a significantly lower content of N than the other composts, whereas N content increased with the proportion of added CS for the other mixtures, being mixture 4 significantly richer in N than mixture 1 (Fig. 5b). All the composts presented more than 1.5% N content, and thus can be classified as high-quality composts following the classification by Diaz et al. 2007 [19].
The C/N ratios measured for the mixtures ranged from 18 to 30 (Fig. 3c). Commonly, C/N ratio is assumed to decrease from the optimal value of 25 for the starting pile to 15 at the final stage [19]. The use of low C/N ratio amendments poses a phytotoxicity risk favouring the conversion of NH4+ into NH3 and/or the release of organic acids due to the immaturity of compost. At the same time, when compost with a high C/N ratio is added to soil, the microbial population tends to compete with plants for soil N, leading to the so-called “nitrogen starvation” [30]. The typical targets for the C/N ratio of composting mixtures could not be applied for blended biochar composts because of the high stability of most biochar C, meaning that some of the C pools are not really involved in the microbial metabolism of the compost; Khan et al. [29] suggested that in matured co-composted biochar the value of C/N can be higher than 21, as in the present study.
Namely, 85–90% of the total N content in composting mixtures is organic (e.g., proteins, simple peptides nucleic acids), and only 10–15% is inorganic N forms immediately available to the plants [19]. During composting, the organic N fraction undergoes microbial mineralization, a two-stage process: the ammonification of complex organic nitrogen to inorganic ammonia leading to the accumulation of NH4+, followed by the nitrification or microbial oxidation of ammonia (NH3) via nitrite (NO2−) to nitrate (NO3−) [31].
The low ammonium concentration in mixture 1 and 2 at the beginning of composting (Fig. 4a) can be explained by the low starting concentration of CS and by the greater efficiency of the system in transforming NH4+ to NO3− especially for the mixture 2, since it has been proved that the BC presence provides a favourable microenvironment for nitrifying bacteria [24]. Mixture 3 exhibited the lowest initial concentration of ammonium, a marked peak raising to 13.6 g kg−1 at around day 30, and a final drop after 30 days to 0.09 g kg−1 (Fig. 4a). The ammonification peak usually reached in the first stages of composting corresponds with the period of maximum biodegradation activity. The high NH4+ peak registered at day 30 can be related to the 10 °C rise recorded as the outcome of the microbial activity growth (Fig. 1a). For mixture 4, ammonia-like odours were noticed during the aeration procedures carried out. This may be attributable to the physical and chemical characteristics of CS presented at high doses in this mixture. On the one hand, N contents of the mixtures increased with the proportion of added CS; on the other hand, CS has a great WHC and a compaction behaviour when humid, favouring the onset of anaerobic spots, provoking N-loss via ammonia, nitrogen oxides (NOx), and nitrous oxide (N2O) emissions [31].
Nitrate concentration for all the mixtures showed an upward trend throughout the first phases of the composting process (Fig. 4b), reaching more than 6 g kg−1 NO3− for mixtures 1 and 2, more than 9 g kg−1 for mixture 4, and more than 16 g kg−1 for mixture 3 in their final composts (Fig. 4b). The declining NH4+ and the proportional increment of NO3− were in line with the steady evolution of the curing stage; indeed, nitrification occurs predominantly at mesophilic temperatures (20–35 °C) [31]. This trend, together with the NH4+/NO3− ratio < 0.16 registered for all the mixtures is an index of high quality amendments and confirms that the compost reaches the maturation phase [29].
Several authors agreed that passive methods such as adsorption by adding BC to compost is an effective method of reducing nitrogen losses [24, 29]. Indeed, BC absorbs/adsorbs NH3, NO3− and NH4+, and a wide range of organic N compounds, reducing greenhouse gasses emission and improving fertiliser properties of compost [21]. Thus, the use of BC constitutes a solution for excessive compaction, anaerobiosis and N losses for those composting mixtures aiming to high N contents, even using higher proportion of CS than those evaluated by the present work.
Agrochemical Quality of the Final Composts
CS is a by-product of the food chain and its content in trace elements is strictly controlled. Therefore, the contents of micro-, macro- and trace elements of the final composts match the requirements of the Spanish national regulation on compost quality (Royal Decree 506/2013) to be classified as Class A amendments (Table 3).
All four composts showed optimal values of P and very high values of K, compared to the majority of compost from vegetal by-products, which gives even more agronomic value to the final composts. High contents of micronutrients such as Ca, Fe, and Mg have also been detected. The range of almost all nutrients increased with the proportion of CS in the initial mixture. However, a further increase of CS proportion above the level established for mixture 4 could exceed the legal limit established for Cu contents in compost. Cu is a crucial micronutrient for plants, but also a plant chlorosis trigger due to its interference with redox and free radical reactions [32]. However, the Cu mobility could be limited by the basic pH [33] of the composts, diminishing any possible risk of contamination.
WHC of our four mixtures accounts for 351, 237, 305, and 335%, respectively (Table 3); such elevated values are due to the fact that CS has more WHC than other similar plant by-products like cereal husk and straws [8]. Applying those amendments to soils will likely increase the storage of water at the soil surface, leading to consequent benefits for plant development and soil structure [34].
Germination test (Table 4) confirmed that the CS has a phytotoxic effect probably mediated by Chlorogenic acid [17] which discourages its use in agriculture as an amendment or mulching agent. However, the resulting compost by the four mixtures (Table 4) did not presented phytotoxicity and even exhibited phytostimulant properties, especially compost 2 and 4. The phytotoxicity reduction confirmed that on the one hand, the mixtures reached full maturity regarding the N cycle; on the other hand, that the decomposition of potentially phytotoxic organic substances of CS, mainly phenolic compounds and Chlorogenic acid has been fulfilled after composting. These results allow the safe application of the four composts to the soil [22].
Potential of Recycling Coffee Silverskin in Agriculture Though Composting
Our results confirm that composting is a low-cost, feasible, and effective method to recycle CS, generating high-value amendment rich in N and K from this by-product. Trace metals contents in CS are far below the levels of concern and CS phytotoxicity is reduced through composting.
CS is a food-grade by-product with low safety issues, easy to collect during the whole year since most of the roasting processes are comparable and insensitive to seasonal variability. Thus the mixing proportions for the composting piles require few if no adjustment once they have been established, easing the handling and the logistic of the whole process.
Europe was the most significant green coffee importer region with 3.9 million tons in 2020 (FAOSTAT, 2022) thus, accounting for the production of ~ 167 thousands of tons of CS during the roasting process. Wong et al. [31] calculated that N losses during composting ranged between 18 and 41% for raw materials comparable to CS and in similar composting conditions. Based on this and on the results of our study, composting the whole CS produced in Europe will lead to a recovery of 2420–3481 N tons, depending on the amount of N loss during the composting process. Similarly, considering that we have observed no-leaking from the composting mixtures, we can assume that composting the whole CS produced in Europe will lead to 1874 K tons, not to mention other micro and macro-nutrients. The data assume a greater relevance considering that composting the CS accumulated per year could account for the 0.01–0.02% of total N fertilizer demand, and 0.05% of K2O fertilizer demand in Europe (FAOSTAT, 2022), reducing the reliance on mineral fertilizers thus contributing to the challenge of meeting the growing demand of food without compromising the environment.
Promoting eco-friendly management of agricultural by-products appears to be essential in the context of the emergency of global warming, helping to reduce greenhouse gas (GHG) emission, the reliance on mineral fertilizers, and achieving the EU landfill reduction target of 10% by 2030 [3].
CS-based soil composts must be considered as amendments increasing the WHC of the soils. The addition of these types of composts is useful to retain higher water quantities over a longer period of time on soil surface, increasing the permeability of water and favouring the formation of soil aggregates, thus improving the overall soil structure and porosity with a fundamental positive effect in regulating the water cycle and protecting soils from erosion and compaction [14].
As suggested by Ronga et al. [16], coffee-derived composts represent a suitable alternative as a growing media ingredient in replacing peat aliquots to produce potted plants. Further studies are needed to assess the potential of BC to increase the proportion of coffee by-products added to a composting pile, since the combination of those materials seems to provide optimum water and air holding capacity, low bulk density, and high cation exchange capacity reducing to a minimum the risk of N losses [35].
Conclusions
Co-composting coffee silverskin with carbon-rich bulking agents as pruning waste and biochar would allow zero-waste in the coffee roasting industry, transforming organic waste into value-added products, thus reaching the circular economy models outlined by the European Union with the Green Deal.
The results obtained in the study suggest that starting the composting process with a 1:1 ratio of CS:co-substrate adding the extra doses of Cs with the ongoing process ensures the recommended exposure time of two weeks at 55 °C during the thermophilic phase, maximizing the amount of compostable CS. Moreover, the addition of BC in blend reduces the risk of compaction of the heap, promoting air flowing and improving the best condition for aerobic digestion.
The micro and macronutrients contents, in particular N and K, makes the compost obtained particularly interesting as organic amendments with a high agronomic value that can be used harmlessly due to their phytostimulant behaviour in germination tests. The relevantly high water holding capacity suggests that these materials may potentially increase the quantity and the persistence of plant-available water in the soil, improving the water balance of the crops. Further research is needed to understand if coffee silverskin-derived compost is suitable as a peat replacement substrate for horticulture and gardening purposes, reducing the reliance on finite resources and fragile ecosystems such as peat bogs.
Data Availability
The data that support the findings of this study are available from the corresponding and lead authors, upon reasonable request.
Code Availability
The code is available from the corresponding and lead authors, upon reasonable request.
Change history
12 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s12649-024-02509-0
References
Santagata, R., Ripa, M., Genovese, A., Ulgiati, S.: Food waste recovery pathways: challenges and opportunities for an emerging bio-based circular economy a systematic review and an assessment. Jnal clean prod 286, 125490 (2021)
Ghisellini, P., Cialani, C., Ulgiati, S.: A review on circular economy: the expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 114, 11–32 (2016). https://doi.org/10.1016/j.jclepro.2015.09.007
European Commission.: Circular economy action plan. Eur. Commun. 28 (2020). https://doi.org/10.2775/855540
Ico.org.: International Coffee Organization—What’s New (2020)
Janissen, B., Huynh, T.: Chemical composition and value-adding applications of coffee industry by-products: a review. Resour. Conserv. Recycl. 128, 110–117 (2018). https://doi.org/10.1016/j.resconrec.2017.10.001
Costa, A.S.G., Alves, R.C., Vinha, A.F., Barreira, S.V.P., Nunes, M.A., Cunha, L.M., Oliveira, M.B.P.P.: Optimization of antioxidants extraction from coffee silverskin, a roasting by-product, having in view a sustainable process. Ind. Crops Prod. 53, 350–357 (2014). https://doi.org/10.1016/j.indcrop.2014.01.006
Alves, R.C., Rodrigues, F., Nunes, M.A., Vinha, A.F., Oliveira, M.B.P.P.: State of the art in coffee processing by-products, In Galanakis, C.M. (Ed.); Handbook of Coffee Processing By-Products, Academic Press pp 1–26. Academic Press (2017). https://doi.org/10.1016/B978-0-12-811290-8.00001-3.
Ballesteros, L.F., Teixeira, J.A., Mussatto, S.I.: Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 7, 3493–3503 (2014). https://doi.org/10.1007/s11947-014-1349-z
FAOSTAT.: Food and Agriculture Organization of the United Nations (FAO): Food and agriculture data (Accessed 10th of March 2022), https://www.fao.org/faostat/en/#home
Bertolino, M., Barbosa-Pereira, L., Ghirardello, D., Botta, C., Rolle, L., Guglielmetti, A., Borotto Dalla Vecchia, S., Zeppa, G.: Coffee silverskin as nutraceutical ingredient in yogurt: its effect on functional properties and its bioaccessibility. J. Sci. Food Agric. 99, 4267–4275 (2019). https://doi.org/10.1002/jsfa.9659
Garcia-Serna, E., Martinez-Saez, N., Mesias, M., Morales, F.J., del Castillo, M.D.: Use of coffee silverskin and stevia to improve the formulation of biscuits. Polish J. Food Nutr. Sci. 64, 243–251 (2014). https://doi.org/10.2478/pjfns-2013-0024
del Pozo, C., Rego, F., Yang, Y., Puy, N., Bartrolí, J., Fàbregas, E., Bridgwater, A.V.: Converting coffee silverskin to value-added products by a slow pyrolysis-based biorefinery process. Fuel Process. Technol. 214, 106708 (2021). https://doi.org/10.1016/j.fuproc.2020.106708
Rodrigues, F., Palmeira-de-Oliveira, A., Das Neves, J., Sarmento, B., Amaral, M.H., Oliveira, M.B.P.P.: Coffee silverskin: a possible valuable cosmetic ingredient. Pharm. Biol. 53, 386–394 (2015). https://doi.org/10.3109/13880209.2014.922589
Tittarelli, F., Petruzzelli, G., Pezzarossa, B., Civilini, M., Benedetti, A., Sequi, P.: Quality and agronomic use of compost. Waste Manag Ser. 8, 119–157 (2007). https://doi.org/10.1016/S1478-7482(07)80010-8
González-Moreno, M.A., García Gracianteparaluceta, B., Marcelino Sádaba, S., Zaratiegui Urdin, J., Robles Domínguez, E., Pérez Ezcurdia, M.A., Seco Meneses, A.: Feasibility of vermicomposting of spent coffee grounds and silverskin from coffee industries: a laboratory study. Agronomy (2020). https://doi.org/10.3390/AGRONOMY10081125
Ronga, D., Pane, C., Zaccardelli, M., Pecchioni, N.: Use of spent coffee ground compost in peat-based growing media for the production of basil and tomato potting plants. Commun. Soil Sci. Plant Anal. 47, 356–368 (2016). https://doi.org/10.1080/00103624.2015.1122803
Al-Charchafchi, F., Al-Quadan, F.: Effect of chlorogenic acid on germination and seedling growth, and on the enzymes activity extracted from artemisia herba alba ASSO. Part I: germination and seedling growth. (2010)
Zucconi, F., Forte, M., Monaco, A., De Bertoldi, M.: Biological evaluation of compost maturity. Biocycle 22, 27–29 (1981)
Diaz, L. F., De Bertoldi, M., Bidlingmaier, W. (Eds.).: Compost Science and Technology, Volume 8 (Waste Management) (2007)
Zhang, J., Chen, G., Sun, H., Zhou, S., Zou, G.: Straw biochar hastens organic matter degradation and produces nutrient-rich compost. Bioresour. Technol. 200, 876–883 (2016). https://doi.org/10.1016/j.biortech.2015.11.016
Sanchez-Monedero, M.A., Cayuela, M.L., Roig, A., Jindo, K., Mondini, C., Bolan, N.: Role of biochar as an additive in organic waste composting. Bioresour. Technol. 247, 1155–1164 (2018)
Gómez-Brandón, M., Lazcano, C., Domínguez, J.: The evaluation of stability and maturity during the composting of cattle manure. Chemosphere 70, 436–444 (2008). https://doi.org/10.1016/J.CHEMOSPHERE.2007.06.065
Madejón, E., Panettieri, M., Madejón, P., Pérez-de-Mora, A.: Composting as sustainable managing option for seaweed blooms on recreational beaches. Waste Biomass Valoriz. 1, 1–13 (2021). https://doi.org/10.1007/s12649-021-01548-1
Kammann, C.I., Schmidt, H.P., Messerschmidt, N., Linsel, S., Steffens, D., Müller, C., Koyro, H.W., Conte, P., Stephen, J.: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 5, 1–13 (2015). https://doi.org/10.1038/srep11080
Verheijen, F.G.A., Graber, E.R., Ameloot, N., Bastos, A.C., Sohi, S., Knicker, H.: Biochars in soils: new insights and emerging research needs. Eur. J. Soil Sci. 65, 22–27 (2014). https://doi.org/10.1111/ejss.12127
Machado, R.M.A., Serralheiro, R.P.: Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 3, 30 (2017). https://doi.org/10.3390/horticulturae3020030
Maas, E.V., Hoffman, G.J.: Crop salt tolerance—current assessment. J. Irrig. Drain. Div. 103, 115–134 (1977). https://doi.org/10.1061/JRCEA4.0001137
Lehmann, J., Joseph, S. (Eds.).: Biochar for environmental management: Science, technology and implementation (2nd ed.). Routledge (2015). https://doi.org/10.4324/9780203762264
Khan, N., Clark, I., Sánchez-Monedero, M.A., Shea, S., Meier, S., Qi, F., Kookana, R.S., Bolan, N.: Physical and chemical properties of biochars co-composted with biowastes and incubated with a chicken litter compost. Chemosphere 142, 14–23 (2016). https://doi.org/10.1016/j.chemosphere.2015.05.065
Azim, K., Soudi, B., Boukhari, S., Perissol, C., Roussos, S., Thami Alami, I.: Composting parameters and compost quality: a literature review. Org. Agr. 8, 141–158 (2018). https://doi.org/10.1007/s13165-017-0180-z
Wong, J.W.C., Wang, X., Selvam, A.: Improving compost quality by controlling nitrogen loss during composting. In: Wong, J.W.-C., Tyagi, R.D, Pendey, A. (Eds.): Current Developments in Biotechnology and Bioengineering: Solid Waste Management, pp. 59–82. Elsevier (2017)
Yruela I.: Copper in plants: acquisition, transport and interactions. Funct Plant Biol. 36(5), 409–430 (2009). https://doi.org/10.1071/FP08288
Abd El-Azeem, S.A.M., Ahmad, M., Usman, A.R.A., Kim, K.R., Oh, S.E., Lee, S.S., Ok, Y.S.: Changes of biochemical properties and heavy metal bioavailability in soil treated with natural liming materials. Environ. Earth Sci. 70, 3411–3420 (2013). https://doi.org/10.1007/S12665-013-2410-3
Domínguez, M.T., Panettieri, M., Madejón, E., Madejón, P.: Thistle crops in marginal lands after compost addition: plant biomass and effect on soil physical, chemical and biological properties. L. Degrad. (2020). https://doi.org/10.1002/ldr.3510
Jindo, K., Sánchez-Monedero, M.A., Mastrolonardo, G., Audette, Y., Higashikawa, F.S., Silva, C.A., Akashi, K., Mondini, C.: Role of biochar in promoting circular economy in the agriculture sector. Part 2: a review of the biochar roles in growing media, composting and as soil amendment (2020)
Acknowledgements
The authors want to thank Ms. Cristina Gómez Ruano, Mr. Héctor Fritis Aguilera and Dr. Asier Goñi Urtiaga for their technical support. Café Candelas SL is gratefully acknowledged for providing the coffee silverskin.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work was financially supported by the Comunidad de Madrid and the Spanish National Research Council (CSIC) through the research grant Atracción de Talento (2019T1/AMB14503).
Author information
Authors and Affiliations
Contributions
Based on the CASRAI’s CRediT definitions of contributor roles, the authors contributed to this work as follow: GP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing—original draft. CP: Data Curation, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review and editing. EM: Investigation, Methodology, Supervision, Validation, Writing—review and editing. MP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—review.and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or proprietary interest in any material discussed in this article.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Picca, G., Plaza, C., Madejón, E. et al. Compositing of Coffee Silverskin with Carbon Rich Materials Leads to High Quality Soil Amendments. Waste Biomass Valor 14, 297–307 (2023). https://doi.org/10.1007/s12649-022-01879-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01879-7