Abstract
This study investigated the effects of peat (PT) and maize straw biochar (MSB) on gas emissions and microbial metabolism characteristics during chicken manure (CM) and maize straw (MS) composting. Three treatments with different additives (0%, 5% PT, 5% MSB added on dry weight basis) were designed to conduct 30-day aerobic composting experiments in nine insulated polyvinyl chloride (PVC) reactors. The results showed that PT and MSB addition increased the temperature and nitrate-nitrogen (NO3−–N) content but decreased the ammonium nitrogen (NH4+–N) content of compost. Compared with control, the total emissions of methane (CH4), nitrous oxide (N2O) and ammonia (NH3) in PT and MSB were reduced by 20.13–30.57%, 28.88–47.46% and 37.35–52.71%, respectively. In addition, PT and MSB amendments improved the microbial utilization capacity on carbohydrates, esters and carboxylic acids. Redundancy analysis revealed that temperature, NH4+–N, pH and microbial metabolism were positively correlated with CH4, N2O and NH3 emissions. Meanwhile, temperature, NH4+–N and pH also had positive correlations with microbial metabolism. Together these results indicated that PT and MSB amendment improved the metabolism capacity of microbes and reduced CH4, N2O and NH3 emissions, eventually mitigating nitrogen loss and promoting the quality of compost product.
Graphical Abstract

Similar content being viewed by others
Statement of Novelty
This experiment aimed to evaluate the efficiency of peat (PT) and maize straw biochar (MSB) to decrease gas emissions of CH4, N2O and NH3 during chicken manure (CM) and maize straw (MS) composting. The carbon sources degradation capacity of microbes at different composting stages was detected. Redundancy analysis revealed the relative contributions of environmental factors and microbial metabolism on gas emissions. Our finding suggested that PT and MSB effectively inhibited the release of CH4, N2O and NH3 and promoted the quality of compost, thus reducing the risk of gas emissions during CM and MS composting and improving the agricultural value of compost products.
Introduction
Chicken manure (CM) has increased rapidly due to the sustained development of the large-scale livestock industry in the past decades. It is estimated that approximately 457 million tons of CM are globally produced each year [1]. CM is a vital fertilizer resource for farming and gardening [2] because it is rich in organic matter and essential plant nutrients such as nitrogen, phosphorous, calcium and potassium [3, 4]. However, CM contains many pathogens, parasite ova, and toxic substances [1], and the direct application of CM into the soil would cause a series of environmental and social problems [5, 6].
Composting has proven to be an eco-friendly and economically feasible technology for recycling organic waste [7]. It is a natural biological process that converts organic matter into stable and sanitary end products [8]. Notably, large amounts of ammonia (NH3) and greenhouse gas (GHG) are released with the decomposition of organic matter during the composting process, resulting in atmospheric pollution and reduction in the agricultural value of compost products. Numerous studies have demonstrated that additives amendment could effectively alleviate gas emissions during composting. A previous report showed that 10% clay addition reduced methane (CH4) and nitrous oxide (N2O) emissions during pig manure composting [9]. Fukumoto et al. [10] revealed that the addition of nitrite-oxidizing bacteria after the thermophilic phase of composting inhibited NO2− accumulation and thus decreased the emission rate of N2O. Awasthi et al. [11] also found that the emissions of CH4, N2O and NH3 were effectively reduced during co-composting of dewatered fresh sewage sludge, lime and biochar.
Peat (PT) is one of the carbon-based materials produced by the decomposition of the plants under a water-saturated and anoxic condition [12]. It was reported that PT possessed excellent characteristics such as high specific area, high pH range and abundant humic acid [13]. PT could be a potential bulking agent for composting because it can increase cation exchange capacity and regulate the moisture content and density of compost [14]. Chang et al. [15] found that the addition of woody peat could promote the maturity process and significantly decrease the nitrogen loss during vegetable waste composting. As a stable carbon-rich material, biochar can regulate bulk density, water retention and oxygen supply during composting [16, 17]. The high porosity and sorption capacity of biochar can provide suitable habitats for microorganisms and stimulate the activity of microbes in compost, which may effectively control nitrogen loss and gas emissions [18, 19]. Awasthi et al. [20] studied the effects of bamboo biochar on poultry manure composting, and their results suggested that bamboo biochar amendment reduced the emissions of GHG and NH3. Agyarko-Mintah et al. [21] found that green waste and poultry litter biochar addition reduced the total nitrogen (TN) and NH3 losses and improved the fertility of final compost products. Wheat straw biochar was demonstrated to play a positive role in organic matter degradation and nitrogen conservation during sewage sludge composting [22, 23]. Compared to wheat straw biochar, maize straw biochar (MSB) performed better in terms of thermophilic temperature and TN concentration during pig manure composting [24]. Although many researches about the influence of biochar as an additive on compost had been reported, no study focused on the contrast effects of PT and MSB on gas emissions and microbial metabolism during CM and MS composting.
In this study, we evaluated the impacts of PT and MSB on CH4, N2O and NH3 emissions during co-composting of CM with MS. Biolog EcoPlate is a low-cost, convenient and rapid technique for detecting the carbon sources utilization ability of microbial populations [25]. Therefore, we used Biolog EcoPlate to investigate the carbon sources metabolism of microbes during composting. The relationships between environmental factors, microbial metabolism and gas emissions were also evaluated to understand the variations of gas emissions during composting. The results obtained from this study would provide new insights into the development of composting technology and better understand important factors responsible for the changes in CH4, N2O and NH3 emissions during the CM and MS composting process.
Materials and Methods
Experimental Design
The composting materials in the present study comprised fresh CM, MS, PT and MSB. The fresh CM and MS were collected from a local farm (Jiujiang, Jiangxi Province, China). The MS was cut into 5 mm pieces before use. PT and MSB were purchased from a factory in Jiangsu Province, China. The biochar was prepared from MS by pyrolysis at a temperature of 400 °C in oxygen-poor condition for 8 h. The basic properties of the raw materials are shown in Table 1. Composting was conducted in 9 insulated polyvinyl chloride (PVC) reactors (diameter 45 cm, height 73 cm, active volume 100 L) [20, 26]. The CM and MS were thoroughly mixed with the ratio of 3:2 (dry weight basis), and each treatment contained approximately 75 L of the mixture. PT or MSB was added in different treatments. The treatment contained 5% (w/w) PT was labeled as PT, while that contained 5% (w/w) MSB was labeled as MSB. The control treatment (CK) was conducted by introducing the mixture without additives. Each treatment was repeated three times. The duration of the composting was 30 days. Deionized water was added to composting piles to maintain 60% moisture content throughout the experiment. Intermittent aeration (twice a day for 20 min) was employed with an air pump at a rate of 0.45 L kg−1 (dry weight) h−1. The pile temperature and ambient temperature were monitored daily using digital thermometers located in the middle of each pile and outside the reactors.
Sampling and Chemical Analysis
Compost samples were collected from each treatment on day 1, 5, 10, 15, 20, 25 and 30. Each sample (about 250 g) was distributed into two parts: one part was immediately stored at 4 °C, and the other was air-dried and preserved in a desiccator. Electricity conductivity (EC) and pH were analyzed using aqueous extracts of compost samples. The aqueous extracts were obtained by mechanically shaking the mixture of samples and deionized water at a ratio of 1:5 (w/v, dry weight basis) for 1 h [27, 28]. TN content was determined by an elemental analyzer (Elementar, Germany) [29]. The concentrations of ammonium nitrogen (NH4+–N) and nitrate-nitrogen (NO3−–N) were measured via sodium hydroxide solution titration and ultraviolet spectrophotometer colorimetry, respectively [30]. The germination index (GI) of samples was analyzed as described previously [31].
Gas Emissions Measurement
Gas sampling was carried out every 5 days during the composting period. Gas samples were collected with syringes and stored in 1-L gas sampling bags [32]. The concentrations of N2O and CH4 in gas samples were analyzed by gas chromatography (Agilent 6890 N, USA) [33]. The NH3 concentration was determined by absorbing the exhausted gas with the boric acid solution before titration with 1 mol L−1 HCl [34].
Biolog EcoPlate Analysis
Biolog EcoPlate analysis was conducted to investigate the capacity of microorganisms to oxidize different carbon sources during composting [35]. The analyses were performed from fresh compost samples collected on day 1, 10 and 30. A fresh compost sample (1 g) was shaken in 100 mL of sterile NaCl solution (0.85%, w/v) for 20 min at 20 °C. The obtained suspension was diluted to 1:1000 and then inoculated onto a Biolog EcoPlate with a pipettor. Each well of the EcoPlate was added with 150 μL of the diluted suspension. The plates were subsequently incubated at 25 °C in the dark for 6 days. The absorbances of the plates were measured at 590 nm and 750 nm every 24 h using an automatic microorganism identification instrument (Biolog, USA). The absorbance data were used to calculate average well color development (AWCD), which could reflect the metabolic capacity of microorganisms in compost. The well absorbance values were corrected by subtracting the absorbance of the control well before the data analysis. The AWCD was calculated as follows: AWCD = Σ ODi/n, where ODi represents the mean optical density value from each well, n is the number of carbon substrates (31 for EcoPlate) [36].
Statistical Analysis
Statistical analysis was conducted using SPSS 16.0 (SPSS for Windows, version 16.0, USA) with the significance level p < 0.05. Redundancy analysis was performed using the R Cluster package (version 3.5.0 for Windows) to identify the correlation between gas emissions, environmental factors and microbial metabolism.
Results and Discussion
Variation in Physicochemical Properties of the Compost
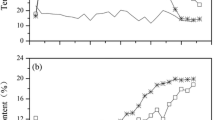
Temperature is considered a principal parameter that determines the evolution of microbial communities and the degradation of organic materials during the composting process [37]. In the present study, the temperature of all piles elevated rapidly during the initial stage of composting (Fig. 1a). It took 5 days for CK to exceed 50 °C, and the following thermophilic phase lasted 6 days. Both PT and MSB exceeded 50 °C on day 4 and maintained the thermophilic phase for 8 days and 9 days, respectively. The peak temperatures of PT (60.5 °C) and MSB (63.2 °C) were higher than that of CK (57.5 °C). As the composting progressed, the temperatures in all groups gradually decreased to close to the ambient level. These results indicated that the addition of PT and MSB increased the temperature and prolonged the thermophilic period of composting. As described in previous studies, the high surface area of PT and MSB could provide more suitable conditions for microbial reproduction and metabolism [13, 38, 39], thus accelerating the degradation of organic matter and increasing the pile temperature. It was reported that temperature above 50 °C could accelerate the rate of composting and kill pathogenic microorganisms [40, 41]. Consequently, the addition of PT and MSB could promote composting maturity and improve the quality of matured compost.
As shown in Fig. 1b, the pH in three treatments rose rapidly in the first 5 days of composting. The mineralization of organic matters such as proteins and amino acids led to the accumulation of NH3 [42], which contributed to the increase of pH during this stage. The peak pH in MSB(8.5), PT(8.3) and CK(8.2) was recorded on day 5, and then the pH gradually decreased in the later stage of composting. The highest pH value in MSB was likely due to the absorption capability of biochar to nitrogen-containing substances during composting [43]. The formation of lower molecular weight fatty acids and CO2 may be responsible for the subsequent decline in pH [44]. At the end of composting, the pH in three treatments was relatively stable, and the pH values were 7.5, 7.6 and 7.8 for CK, PT and MSB, respectively.
EC can reflect the compost salinity and determine the effects of matured compost on seed germination [45]. The initial EC values in three treatments ranged from 4.3 to 4.6 mS cm−1 (Fig. 1c). The EC value of each treatment decreased sharply in the early stage of composting. From day 5 onward, the EC values in three treatments gradually increased and then fluctuated until the end of composting. The final EC values in CK, PT and MSB were 3.3, 2.8 and 2.9 mS cm−1, respectively. In general, composting products could be safely applied into farmland only when the EC values of products were lower than 4.0 mS·cm−1 [46]. Hence, the end-products in all treatments met the safety standards of application.
Evolution of NH4 +–N, NO3 −–N and TN During Composting
As shown in Fig. 2a, the NH4+–N content of three treatments increased rapidly during the first 5 days. The increase in NH4+–N was likely due to the rapid decomposition of organic nitrogenous compounds in the early stage of composting [47]. Fang et al. [48] pointed out that the low pH in compost promoted the conversion of NH3 into NH4+–N, which could explain the higher NH4+–N content in CK compared to PT and MSB during this period. The peak NH4+–N contents were 3.55, 3.11 and 2.81 g kg−1 in CK, PT, and MSB treatments, respectively. Afterward, the NH4+–N content in three treatments sharply declined from day 5 to day 10 and then gradually stabilized. Raj and Antil [49] reported that the decrease of the NH4+ might enhance nitrification and immobilization by microorganisms. After 30 days of composting, the NH4+–N contents for CK, PT and MSB were 0.38, 0.18 and 0.08 g kg−1, respectively. As described previously, the NH4+–N content lower than 0.20 g kg−1 was regarded as a maturity and stability index for compost [8]. Our data indicated that the addition of PT and MSB could improve the maturity and stability of final composting products.
NO3−–N is an essential nitrogen source for plant metabolism and growth [50]. In the present study, a similar trend of NO3−–N content was observed in all treatments (Fig. 2b). The NO3−–N content in three treatments slightly decreased during the initial stage and then gradually increased. Previous studies have demonstrated that the nitrifying bacteria could transform NH3 into NO3−–N during composting, and high temperature strongly inhibited the growth and activity of nitrifying bacteria [51, 52]. Therefore, the increase of NO3−–N content mainly occurred during the mature stage of composting. At the end of composting, the NO3−–N contents were 0.52, 0.64 and 0.57 g kg−1 for CK, PT and MSB treatments, respectively. It was noted that PT exhibited higher content of NO3−–N than CK and MSB. As the first step of nitrification, oxidization of NH3 needed a suitable amount of alkali [53], and the decrease of pH would be favorable for the nitrification process. We inferred that the acidity of wood peat might provide a more suitable pH condition for nitrification and therefore increase the NO3−–N content during composting.
TN is one of the primary parameters to evaluate the quality of compost products [8]. The TN content of all treatments slightly decreased in the first 5 days and then gradually increased over time. The variation of TN content was possibly related to the continuous degradation of nitrogenous compounds during composting [54]. The TN content in end-products of CK, PT and MSB were 28.23, 32.77 and 29.83 g kg−1, respectively. This finding suggested that the addition of PT and MSB could increase the nutrient contents of compost products. Awasthi et al. [27, 28] reported that the additives amendment facilitated the growth and activity of aerobic bacteria associated with organic nitrogen mineralization and therefore increased the nutrient contents of compost, which was consistent with our results.
Changes in Greenhouse Gases and Ammonia Emissions
CH4 is produced by methanogens under anaerobic conditions [34]. The CH4 emissions of all treatments primarily occurred in the thermophilic stage and then gradually decreased (Fig. 3a). With the increase of temperature, large amounts of organic matter were rapidly decomposed by microorganisms and caused inadequate oxygen supply during the thermophilic stage of composting [55, 56]. As a result, the partially anaerobic area in compost was developed in this stage to produce CH4. Besides, Gilroyed et al. [57] pointed out that methanotrophic community was not established in the initial phase of composting and thus, the oxidation of CH4 was not completely efficient. The cumulative CH4 emission in three treatments showed an increase in the early stage and then tended to be stable (Fig. 3b). After 30 days of composting, the addition of PT and MSB reduced the total CH4 emission by 20.13% and 30.57% compared with CK, respectively. It was possible because the high specific area of PT and MSB could partially improve the aeration condition, thereby accelerating the growth and activity of methanotrophs and enhancing CH4 oxidation [13, 58]. In addition, the high pore volume of biochar had been demonstrated to increase CH4 sorption [59], which may account for the highest removal rate in compost with PT addition.
Many researchers have studied the N2O emission profile for its profound impacts on nitrogen conservation and global warming [47, 60]. In the present study, all treatments exhibited a relatively high N2O emission rate in the early stage of composting and reached the highest N2O emission in the first week (Fig. 3c). The peak values of N2O emission in CK, PT and MSB were 2.97, 2.57 and 2.18 g kg−1 day−1, respectively. As reported by previous studies, the N2O emission during composting is a complex process influenced by many factors, and N2O can be produced under both nitrification and denitrification processes [61,62,63]. Notably, the activity of nitrifiers was generally considered to be inhibited when the temperature was above 40 °C [64]. Meanwhile, Philippe et al. [65] reported that rapid depletion of oxygen and low nitrate concentration promoted the formation of N2O in the denitrification process. These findings suggested that the large amount of N2O in the first week might be produced mainly through the denitrification of nitrate. The high CH4 emission in the first week (Fig. 3a) further demonstrated the inadequate oxygen supply during this period, supporting the possibility of N2O as a by-product of denitrification. As shown in Fig. 3d, the cumulative emissions of N2O in PT and MSB remained constant after day 17, while that in CK reached a stale value until day 26. Compared with CK, the cumulative emissions of N2O on day 30 were reduced by 28.88% and 47.46% in PT and MSB, respectively. These results indicated that PT and MSB, especially MSB, efficiently decreased the N2O emission during CM and MS composting. The significant difference between MSB and CK was supported by Cornelissen et al. [66], who found direct sorption of N2O into biochar. Joseph et al. [67] also reported that biochar had redox activity and might act as the reducing agent in soil. Given the possibility of N2O emission via denitrification, we supposed that the redox activity of MSB might facilitate the transformation of NO3−–N to N2, thus decreasing the N2O emission during composting.
The NH3 emission during composting causes nitrogen loss of compost products [6]. The variations of NH3 emission in our experiment are shown in Fig. 3e. Initially, the NH3 emissions in the three treatments increased sharply and reached their maximum values in the thermophilic stage. The peak values were 0.75, 0.52 and 0.59 g kg−1 day−1 for CK, PT and MSB, respectively. The emission of NH3 in animal manure was affected by pH and NH4+–N H3 transformation equilibrium [54]. In the initial stage, nutrient degradation was accompanied by increased pH and temperature in compost (Fig. 1). Meanwhile, the mineralization and ammoniation of organic nitrogen rapidly increased the production of NH4+–N, which could volatilize in the form of NH3 due to high temperature and pH [68]. After 7 days of composting, the NH3 emission gradually decreased and eventually approached zero. This was attributable to the decrease of pH, which increased the content of acid radical ions and shifted the NH4+–N H3 equilibrium [69]. The total cumulative NH3 emissions were 1.57 and 2.08 g kg−1 for PT and MSB, respectively, which were lower than CK (3.32 g kg−1). The results showed that the addition of PT and MSB could efficiently reduce the volatilization of NH3 during composting, which might be associated with the large surface area and small particle size of PT and MSB. Previous research demonstrated that bulking agent with a large specific surface area could absorb NH4+–N and NH3 [32]. Besides, PT and MSB with small particle sizes could result in high bulk density of mixed compost materials, thereby reducing the NH3 emission [62].
Carbon Sources Utilization Capacity of Microbial Community
Biolog EcoPlate analysis was conducted to evaluate the carbon sources utilization capacity of microbial community at different stages of composting. The 31 carbon sources in the Biolog EcoPlate were classified into six categories according to the biochemical properties. The AWCD of six carbon sources in three treatments is shown in Fig. 4. The AWCDs of carbohydrates, amino acids, esters and carboxylic acids increased in PT and MSB compared to CK on day 1, indicating that the addition of PT and MSB improved the carbon sources utilization ability of microbes. The peak values of six carbon sources for three treatments were observed on day 10, which meant that the microbial community had the highest metabolic activity in the thermophilic phase among the three composting stages. On day 30, the AWCDs of most carbon sources were still higher in PT and MSB than those in CK. PT and MSB provided more nutrients and suitable habitats for microorganisms and consequently increased the metabolic activity of microbial community. As reported in a previous study, microbial activity of compost exerted a certain role in controlling soil-borne pathogens [70]. Therefore, compost with the addition of PT or MSB might be more efficient at maintaining soil health.
Relationships Among Environmental Factors, Microbial Metabolism and Gas Emissions
Redundancy analysis was performed to determine the relationships among environmental factors, microbial metabolism and gas emissions. The results showed that the selected variables accounted for 93.89% of the total variations in gas emissions (Fig. 5). Among the selected factors, environmental factors and microbial metabolism explained 51.10% and 47.46% of the variations, respectively. Environmental factors such as pH, NH4+–N and temperature had positive correlations with CH4 emission. NH4+–N was negatively correlated with NH3 emission, because NH4+–N and NH3 would be transformed to each other under suitable condition [6]. Similarly, Yang et al. [71] indicated a negative correlation between NH4+–N and NH3 during pig manure composting. Apart from NH3, NH4+–N also showed a negative correlation with N2O emission. The reason for this may be that the higher NH4+–N concentration restricted the activity of nitrifying bacteria and therefore caused insufficient NO3−–N to produce N2O. Moreover, EC exhibited negative correlations with the emissions of N2O and NH3. Previous studies demonstrated that the increase of soluble salts concentration in compost could lead to the increase of EC [23, 72]. The emissions of N2O and NH3 reduced the concentration of soluble nitrogen salts and consequently resulted in the decrease of EC in this experiment. Interestingly, our data showed that pH was negatively correlated with the N2O emission. Many reports have confirmed that pH could directly affect N2O production by regulating nitrification and denitrification processes [73, 74]. The N2O-reductase has been recognized as the key enzyme for reducing N2O production in denitrification [75]. It is noteworthy that the decrease of pH would inhibit the activity of N2O-reductase [73], thus leading to higher N2O emissions. Microbial metabolism exhibited an essential role in the emissions of CH4, N2O and NH3. The relationships between microbial metabolism and gas emissions were previously demonstrated. For example, Zhang et al. [76] reported that the reduction in CH4 emission was closely associated with the metabolism of methanogens and methanotrophs. Getahun et al. [77] also pointed out that microbial metabolism could convert some proteins to NH3. In addition, microbial metabolism was positively correlated with pH, NH4+–N and temperature. Overall, the addition of PT and MSB changed environmental factors of compost and further influenced the metabolism of microbial, eventually resulting in the variations of CH4, N2O and NH3 emissions.
Evaluation of Compost Maturity
The GI is considered one of the most sensitive indicators for evaluating changes in product toxicity and the degree of compost maturity [8]. The GI values among all treatments increased gradually throughout the composting period (Fig. 6). At the end of composting, the GI values in CK, PT and MSB were 104.5%, 118.0% and 126.9%, respectively. These results demonstrated that the addition of PT and MSB improved the quality of compost products. According to the standard of U.S. Composting Council, the GI value of compost product should exceed 90%. Therefore, the addition of MSB promoted the quality of compost product better than PT. Guo et al. [78] found that NH4+–N content had negative correlations with GI during composting. The higher GI values in PT and MSB might be due to the reduction of NH4+–N content during the composting period.
Conclusions
This study indicated that PT and MSB amendment increased the pile temperature and prolonged the thermophilic stage of compost. PT and MSB could facilitate the degradation of organic matter and nitrogen conservation during compost. The addition of PT and MSB increased the GI value and promoted the quality of compost. PT and MSB effectively inhibited the release of CH4, N2O and NH3 during co-composting of CM and MS. Biolog EcoPlate analysis revealed that PT and MSB enhanced the microbial metabolism involved in carbohydrates, esters and carboxylic acids throughout the composting period. The emissions of CH4, N2O and NH3 were significantly associated with microbial metabolism, which was influenced by temperature, NH4+–N and pH of compost.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mao, H., Wang, K., Wang, Z., Peng, J., Ren, N.: Metabolic function, trophic mode, organics degradation ability and influence factor of bacterial and fungal communities in chicken manure composting. Bioresour. Technol. 302, 122883 (2020). https://doi.org/10.1016/j.biortech.2020.122883
Omar, L., Osumanu Haruna, A., Majid, N.M.: Effect of organic amendment derived from co-composting of chicken slurry and rice straw on reducing nitrogen loss from urea. Commun. Soil Sci. Plan. 47, 639–656 (2016). https://doi.org/10.1080/00103624.2016.1141919
Neslihan, A.: A systematic review of biochar use in animal waste composting. Waste Manage. 88, 291–300 (2019). https://doi.org/10.1016/j.wasman.2019.03.054
Sugiyama, S., Kitora, R., Kinoshita, H., Nakagawa, K., Katoh, M., Nakasaki, K.: Recovery of calcium phosphates from composted chicken manure. J. Chem. Eng. Jpn. 49, 224–228 (2016). https://doi.org/10.1252/jcej.15we111
Shi, M.Z., Wei, Z., Wang, L., Wu, J., Zhang, D., Wei, D., Tang, Y., Zhao, Y.: Response of humic acid formation to elevated nitrate during chicken manure composting. Bioresour. Technol. 258, 390–394 (2018). https://doi.org/10.1016/j.biortech.2018.03.056
Wang, Q., Wang, Z., Awasthi, M.K., Jiang, Y.H., Li, R.H., Ren, X.N., Zhao, J.C., Shen, F., Wang, M.J., Zhang, Z.Q.: Evaluation of medical stone amendment for the reduction of nitrogen loss and bioavailability of heavy metals during pig manure composting. Bioresour. Technol. 220, 297–304 (2016). https://doi.org/10.1016/j.biortech.2016.08.081
Wang, Q., Awasthi, M.K., Ren, X., Zhao, J., Li, R., Wang, Z., Wang, M., Chen, H., Zhang, Z.: Combining biochar, zeolite and wood vinegar for composting of pig manure: The effect on greenhouse gas emission and nitrogen conservation. Waste Manage. 74, 221–230 (2018). https://doi.org/10.1016/j.wasman.2018.01.015
Bernal, M.P., Alburquerque, J.A., Moral, R.: Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 100, 5444–5453 (2009). https://doi.org/10.1016/j.biortech.2008.11.027
Ren, X., Wang, Q., Li, R., Chang, C.C., Pan, J., Zhang, Z.: Effect of clay on greenhouse gas emissions and humification during pig manure composting as supported by spectroscopic evidence. Sci. Total. Environ. 737, 139712 (2020). https://doi.org/10.1016/j.scitotenv.2020.139712
Fukumoto, Y., Suzuki, K., Osada, T., Kuroda, K., Hanajima, D., Yasuda, T., Haga, K.: Reduction of nitrous oxide emission from pig manure composting by addition of nitrite-oxidizing bacteria. Environ. Sci. Technol. 40, 6787–6791 (2006). https://doi.org/10.1021/es0611801
Awasthi, M.K., Wang, Q., Huang, H., Li, R., Shen, F., Lahori, A.H., Wang, P., Guo, D., Guo, Z., Jiang, S., Zhang, Z.: Effect of biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J. Clean. Prod. 135, 829–835 (2016). https://doi.org/10.1016/j.jclepro.2016.07.008
Orem, W., Finkelman, R.: Coal formation and geochemistry. Treatise Geochem. 7, 191–222 (2003). https://doi.org/10.1016/B0-08-043751-6/07097-3
Mathur, S.P., Daigle, J.Y., Lévesque, M., Dinel, H.: The feasibility of preparing high quality composts from fish scrap and peat with seaweeds or crab scrap. Biol. Agric. Hortic. 4, 27–38 (1986). https://doi.org/10.1080/01448765.1986.9754484
Yuan, J., Zhang, D., Du, L., Yang, F., Li, G., Luo, Y.: Effect of woody peat as an additive on maturity and gaseous emissions during pig manure composting. Compost Sci. Util. 27, 69–80 (2019). https://doi.org/10.1080/1065657X.2018.1507850
Chang, R., Li, Y., Chen, Q., Guo, Q., Jia, J.: Comparing the effects of three in situ methods on nitrogen loss control, temperature dynamics and maturity during composting of agricultural wastes with a stage of temperatures over 70 °C. J. Environ. Manage. 230, 119–127 (2019). https://doi.org/10.1016/j.jenvman.2018.09.076
Ogawa, M., Okimori, Y.: Pioneering works in biochar research, Japan. Aust. J. Soil Res. 48, 489–500 (2010). https://doi.org/10.1071/SR10006
Zhang, L., Sun, X.Y.: Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 171, 274–284 (2014). https://doi.org/10.1016/j.biortech.2014.08.079
Cui, L., Yan, J., Yang, Y., Li, L., Quan, G., Ding, C., Chen, T., Fu, Q., Chang, A.: Influence of biochar on microbial activities of heavy metals contaminated paddy fields. BioResourses 8, 5536–5548 (2013). https://doi.org/10.15376/biores.8.4.5536-5548
Pommier, T., Merroune, A., Bettarel, Y., Got, P., Janeau, J.L., Jouquet, P., Thu, T.D., Toan, T.D., Rochelle-Newall, E.: Off-site impacts of agricultural composting: role of terrestrially derived organic matter in structuring aquatic microbial communities and their metabolic potential. FEMS Microbiol. Ecol. 90, 622–632 (2014). https://doi.org/10.1111/1574-6941.12421
Awasthi, M.K., Duan, Y., Awasthi, S.K., Liu, T., Zhang, Z.: Influence of bamboo biochar on mitigating greenhouse gas emissions and nitrogen loss during poultry manure composting. Bioresour. Technol. 303, 122952 (2020). https://doi.org/10.1016/j.biortech.2020.122952
Agyarko-Mintah, E., Cowie, A., Van Zwieten, L., Singh, B.P., Smillie, R., Harden, S., Fornasier, F.: Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manage. 61, 129–137 (2017). https://doi.org/10.1016/j.wasman.2016.12.009
Malińska, K., Zabochnicka-Świątek, M., Dach, J.: Effects of biochar amendment on ammonia emission during composting of sewage sludge. Ecol. Eng. 71, 474–478 (2014). https://doi.org/10.1016/j.ecoleng.2014.07.012
Zhang, J., Lu, F., Shao, L., He, P.: The use of biochar-amended composting to improve the humification and degradation of sewage sludge. Bioresour. Technol. 168, 252–258 (2014). https://doi.org/10.1016/j.biortech.2014.02.080
Zhou, G., Xu, X., Qiu, X., Zhang, J.: Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour Technol. 272, 10–18 (2019). https://doi.org/10.1016/j.biortech.2018.09.135
Németh, I., Molnár, S., Vaszita, E., Molnár, M.: The biolog EcoPlate™ technique for assessing the effect of metal oxide nanoparticles on freshwater microbial communities. Nanomaterials (Basel) 11(7), 1777 (2021). https://doi.org/10.3390/nano11071777
Chen, H., Awasthi, S.K., Liu, T., Duan, Y., Ren, X., Zhang, Z., Pandey, A., Awasthi, M.K.: Effects of microbial culture and chicken manure biochar on compost maturity and greenhouse gas emissions during chicken manure composting. J. Hazard Mater. 389, 121908 (2020). https://doi.org/10.1016/j.jhazmat.2019.121908
Awasthi, M.K., Wang, Q., Huang, H., Ren, X., Lahori, A.H., Mahar, A., Ali, A., Shen, F., Li, R., Zhang, Z.: Influence of zeolite and lime as additives on greenhouse gas emissions and maturity evolution during sewage sludge composting. Bioresour. Technol. 216, 172–181 (2016). https://doi.org/10.1016/j.biortech.2016.05.065
Awasthi, M.K., Wang, Q., Ren, X., Zhao, J., Huang, H., Awasthi, S.K., Lahori, A.H., Li, R., Zhou, L., Zhang, Z.: Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour Technol. 219, 270–280 (2016). https://doi.org/10.1016/j.biortech.2016.07.128
Zhu-Barker, X., Bailey, S.K., Paw U, K.T., Burger, M., Horwath, W.R.: Greenhouse gas emissions from green waste composting windrow. Waste Manage. 59, 70–79 (2017). https://doi.org/10.1016/j.wasman.2016.10.004
Zhu, F., Hong, C., Wang, W., Lyu, H., Zhu, W., Xv, H., Yao, Y.: A microbial agent effectively reduces ammonia volatilization and ensures good maggot yield from pig manure composted via housefly larvae cultivation. J. Clean. Prod. 270, 122373 (2020). https://doi.org/10.1016/j.jclepro.2020.122373
Yang, F., Li, G.X., Yang, Q.Y., Luo, W.H.: Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 93, 1393–1399 (2013). https://doi.org/10.1016/j.chemosphere.2013.07.002
He, X., Yin, H., Han, L., Cui, R., Fang, C., Huang, G.: Effects of biochar size and type on gaseous emissions during pig manure/wheat straw aerobic composting: Insights into multivariate-microscale characterization and microbial mechanism. Bioresour. Technol. 271, 375–382 (2019). https://doi.org/10.1016/j.biortech.2018.09.104
Yang, F., Li, Y., Han, Y., Qian, W., Li, G., Luo, W.: Performance of mature compost to control gaseous emissions in kitchen waste composting. Sci. Total Environ. 657, 262–269 (2019). https://doi.org/10.1016/j.scitotenv.2018.12.030
Yang, F., Li, G.X., Shi, H., Wang, Y.M.: Effects of phosphogypsum and superphosphate on compost maturity and gas emissions during kitchen waste composting. Waste Manage. 36, 70–76 (2015). https://doi.org/10.1016/j.wasman.2014.11.012
Gomez, E.D., Ferreras, L., Toresani, S.: Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 97, 1484–1489 (2006). https://doi.org/10.1016/j.biortech.2005.06.021
Frąc, M., Oszust, K., Lipiec, J.: Community level physiological profiles (CLPP), characterization and microbial activity of soil amended with dairy sewage sludge. Sensors (Basel) 12, 3253–3268 (2012). https://doi.org/10.3390/s120303253
Liu, W., Wang, S.T., Zhang, J., Xu, T.: Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour. Technol. 154, 148–154 (2014). https://doi.org/10.1016/j.biortech.2013.12.022
Awasthi, M.K., Wang, M., Pandey, A., Chen, H., Awasthi, S.K., Wang, Q., Ren, X., Lahori, A.H., Li, D., Li, R., Zhang, Z.: Heterogeneity of zeolite combined with biochar properties as a function of sewage sludge composting and production of nutrient-rich compost. Waste Manage. 68, 760–773 (2017). https://doi.org/10.1016/j.wasman.2017.06.008
Zhang, L., Zhang, H., Wang, Z., Chen, G., Wang, L.: Dynamic changes of the dominant functioning microbial community in the compost of a 90–m3 aerobic solid state fermentor revealed by integrated meta-omics. Bioresour. Technol. 203, 1–10 (2016). https://doi.org/10.1016/j.biortech.2015.12.040
He, Y., Xie, K., Xu, P., Huang, X., Gu, W., Zhang, F., Tang, S.: Evolution of microbial community diversity and enzymatic activity during composting. Res. Microbiol. 164, 189–198 (2013). https://doi.org/10.1016/j.resmic.2012.11.001
Zhang, L., Sun, X.Y., Tian, Y., Gong, X.Q.: Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 131, 68–75 (2013). https://doi.org/10.1016/j.biortech.2012.10.059
Gigliotti, G., Proietti, P., Said-Pullicino, D., Nasini, L., Pezzolla, D., Rosati, L., Porceddu, P.R.: Co-composting of olive husks with high moisture contents: Organic matter dynamics and compost quality. Int. Biodeterior. Biodegrad. 67, 8–14 (2012). https://doi.org/10.1016/j.ibiod.2011.11.009
Chen, W., Liao, X., Wu, Y., Liang, J., Mi, J., Huang, J., Zhang, H., Wu, Y., Qiao, Z., Li, X., Wang, Y.: Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manage. 61, 506–515 (2017). https://doi.org/10.1016/j.wasman.2017.01.014
Tu, Z., Ren, X., Zhao, J., Awasthi, S.K., Wang, Q., Awasthi, M.K., Zhang, Z., Li, R.: Synergistic effects of biochar/microbial inoculation on the enhancement of pig manure composting. Biochar 1, 127–137 (2019). https://doi.org/10.1007/s42773-019-00003-8
Huang, G.F., Wong, J.W., Wu, Q.T., Nagar, B.B.: Effect of C/N on composting of pig manure with sawdust. Waste Manage. 24, 805–813 (2004). https://doi.org/10.1016/j.wasman.2004.03.011
García, C., Hernández, T., Costa, F.: Study on water extract of sewage sludge composts. Soil Sci. Plant Nutr. 37, 399–408 (1991). https://doi.org/10.1080/00380768.1991.10415052
Awasthi, M.K., Wang, Q., Awasthi, S.K., Wang, M., Chen, H., Ren, X., Zhao, J., Zhang, Z.: Influence of medical stone amendment on gaseous emissions, microbial biomass and abundance of ammonia oxidizing bacteria genes during biosolids composting. Bioresour. Technol. 247, 970–979 (2018). https://doi.org/10.1016/j.biortech.2017.09.201
Fang, M., Wong, J., Ma, K.K., Wong, M.: Co-composting of sewage sludge and coal fly ash: nutrient transformations. Bioresour. Technol. 67, 19–24 (1999). https://doi.org/10.1016/S0960-8524(99)00095-4
Raj, D., Antil, R.S.: Evaluation of maturity and stability parameters of composts prepared from farm wastes. Bioresour. Technol. 102, 2868–2873 (2011). https://doi.org/10.1016/j.biortech.2010.10.077
Vega, A., O’Brien, J.A., Gutierrez, R.A.: Nitrate and hormonal signaling crosstalk for plant growth and development. Curr. Opin. Plant Biol. 52, 155–163 (2019). https://doi.org/10.1016/j.pbi.2019.10.001
Zeng, G., Zhang, J., Chen, Y., Yu, Z., Yu, M., Li, H., Liu, Z., Chen, M., Lu, L., Hu, C.: Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting. Bioresour. Technol. 102, 9026–9032 (2011). https://doi.org/10.1016/j.biortech.2011.07.076
Zhao, Y., Li, W., Chen, L., Meng, L., Zheng, Z.: Effect of enriched thermotolerant nitrifying bacteria inoculation on reducing nitrogen loss during sewage sludge composting. Bioresour. Technol. 311, 123461 (2020). https://doi.org/10.1016/j.biortech.2020.123461
Gujer, W., Jenkins, D.: A nitrification model for the contact stabilization activated sludge process. Water Res. 9, 561–566 (1975). https://doi.org/10.1016/0043-1354(75)90082-2
Li, R., Wang, Q., Zhang, Z., Zhang, G., Li, Z., Wang, L., Zheng, J.: Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 36, 815–826 (2015). https://doi.org/10.1080/09593330.2014.963692
Jiang, T., Schuchardt, F., Li, G.X., Guo, R., Luo, Y.M.: Gaseous emission during the composting of pig feces from Chinese Ganqinfen system. Chemosphere 90, 1545–1551 (2013). https://doi.org/10.1016/j.chemosphere.2012.08.056
Manios, T., Maniadakis, K., Boutzakis, P., Naziridis, Y., Lasaridi, K., Markakis, G., Stentiford, E.I.: Methane and carbon dioxide emission in a two-phase olive oil mill sludge windrow pile during composting. Waste Manage. 27, 1092–1098 (2007). https://doi.org/10.1016/j.wasman.2006.05.012
Gilroyed, B., Hao, X., Larney, F., McAllister, T.: Greenhouse gas emissions from cattle feedlot manure composting and anaerobic digestion as a potential mitigation strategy. ACS Symp. Ser. 1072, 419–441 (2011). https://doi.org/10.1021/bk-2011-1072.ch022
Scheutz, C., Pedicone, A., Pedersen, G.B., Kjeldsen, P.: Evaluation of respiration in compost landfill biocovers intended for methane oxidation. Waste Manage. 31, 895–902 (2011). https://doi.org/10.1016/j.wasman.2010.11.019
Agyarko-Mintah, E., Cowie, A., Singh, B.P., Joseph, S., Van Zwieten, L., Cowie, A., Harden, S., Smillie, R.: Biochar increases nitrogen retention and lowers greenhouse gas emissions when added to composting poultry litter. Waste Manage. 61, 138–149 (2017). https://doi.org/10.1016/j.wasman.2016.11.027
Maulini-Duran, C., Artola, A., Font, X., Sanchez, A.: Gaseous emissions in municipal wastes composting: effect of the bulking agent. Bioresour. Technol. 172, 260–268 (2014). https://doi.org/10.1016/j.biortech.2014.09.041
Awasthi, M.K., Pandey, A.K., Bundela, P.S., Wong, J.W.C., Li, R., Zhang, Z.: Co-composting of gelatin industry sludge combined with organic fraction of municipal solid waste and poultry waste employing zeolite mixed with enriched nitrifying bacterial consortium. Bioresour. Technol. 213, 181–189 (2016). https://doi.org/10.1016/j.biortech.2016.02.026
Chan, M., Selvam, A., Wong, J.: Reducing nitrogen loss and salinity during “struvite” food waste composting by zeolite amendment. Bioresour. Technol. 200, 838–844 (2016). https://doi.org/10.1016/j.biortech.2015.10.093
Santos, C., Guofo, P., Fonseca, J., Pereira, J., Ferreira, L.: Effect of lignocellulosic and phenolic compounds on ammonia, nitric oxide and greenhouse gas emissions during composting. J. Clean. Prod. 171, 548–556 (2018). https://doi.org/10.1016/j.jclepro.2017.10.050
Anthonisen, A., Loehr, R., Prakasam, T., Srinath, E.G.: Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control Fed. 48, 835–852 (1976). https://doi.org/10.2307/25038971
Philippe, F.X., Laitat, M., Nicks, B., Cabaraux, J.F.: Ammonia and greenhouse gas emissions during the fattening of pigs kept on two types of straw floor. Agric. Ecosyst. Environ. 150, 45–53 (2012). https://doi.org/10.1016/j.agee.2012.01.006
Cornelissen, G., Rutherford, D.W., Arp, H.P., Dorsch, P., Kelly, C.N., Rostad, C.E.: Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ. Sci. Technol. 47, 7704–7712 (2013). https://doi.org/10.1021/es400676q
Joseph, S.D., Camps-Arbestain, M., Lin, Y., Munroe, P., Chia, C.H., Hook, J., Van Zwieten, L., Kimber, S., Cowie, A., Singh, B.P., Lehmann, J., Foidl, N., Smernik, R.J., Amonette, J.E.: An investigation into reactions of biochar in soils. Aust. J. Soil Res. 48, 501–515 (2010). https://doi.org/10.1071/SR10009
Luo, W.H., Yuan, J., Luo, Y.M., Li, G.X., Nghiem, L.D., Price, W.E.: Effects of mixing and covering with mature compost on gaseous emissions during composting. Chemosphere 117, 14–19 (2014). https://doi.org/10.1016/j.chemosphere.2014.05.043
Li, Y., Liu, T., Song, J., Lv, J., Jiang, J.: Effects of chemical additives on emissions of ammonia and greenhouse gas during sewage sludge composting. Process. Saf. Environ. 143, 129–137 (2020). https://doi.org/10.1016/j.psep.2020.05.056
Ntougias, S., Papadopoulou, K.K., Zervakis, G.I., Kavroulakis, N., Ehaliotis, C.: Suppression of soil-borne pathogens of tomato by composts derived from agro-industrial wastes abundant in Mediterranean regions. Biol. Fert. Soils 44, 1081–1090 (2008). https://doi.org/10.1007/s00374-008-0295-1
Yang, Y., Awasthi, M.K., Ren, X., Guo, H., Lv, J.: Effect of bean dregs on nitrogen transformation and bacterial dynamics during pig manure composting. Bioresour Technol. 288, 121430 (2019). https://doi.org/10.1016/j.biortech.2019.121430
Huang, G.F., Wu, Q.T., Wong, J.W., Nagar, B.B.: Transformation of organic matter during co-composting of pig manure with sawdust. Bioresour Technol. 97, 1834–1842 (2006). https://doi.org/10.1016/j.biortech.2005.08.024
Qu, Z., Wang, J., Almoy, T., Bakken, L.R.: Excessive use of nitrogen in Chinese agriculture results in high N2O/(N2O+N2) product ratio of denitrification, primarily due to acidification of the soils. Glob. Change. Biol. 20, 1685–1698 (2014). https://doi.org/10.1111/gcb.12461
Xiao, K., Xu, J., Tang, C., Zhang, J., Brookes, P.C.: Differences in carbon and nitrogen mineralization in soils of differing initial pH induced by electrokinesis and receiving crop residue amendments. Soil Biol. Biochem. 67, 70–84 (2013). https://doi.org/10.1016/j.soilbio.2013.08.012
Bakken, L.R., Bergaust, L., Liu, B., Frostegard, A.: Regulation of denitrification at the cellular level: a clue to the understanding of N2O emissions from soils. Philos. Trans. R. Soc. B 367, 1226–1234 (2012). https://doi.org/10.1098/rstb.2011.0321
Zhang, K., Wu, X., Luo, H., Li, X., Chen, W., Chen, J., Mo, Y., Wang, W.: CH4 control and associated microbial process from constructed wetland (CW) by microbial fuel cells (MFC). J. Environ. Manage. 260, 110071 (2020). https://doi.org/10.1016/j.jenvman.2020.110071
Getahun, D., Alemneh, T., Akeberegn, D., Getabalew, M., Zewdie, D.: Urea metabolism and recycling in ruminants. Biomed. J. Sci. Tech. Res. 20, 14790–14796 (2019). https://doi.org/10.26717/BJSTR.2019.20.003401
Guo, R., Li, G., Jiang, T., Schuchardt, F., Chen, T., Zhao, Y., Shen, Y.: Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 112, 171–178 (2012). https://doi.org/10.1016/j.biortech.2012.02.099
Acknowledgements
The authors thank the reviewers for their valuable comments, and the authors thank the editor for his efforts in this paper.
Funding
This work was supported by the National Natural Science Foundation of China (41661068), the Academic Leader Program of Jiangxi Province (20204BCJL23042).
Author information
Authors and Affiliations
Contributions
MF designed the research content, analyzed the data, and wrote the first draft of the paper. XW was involved in the data analysis. XQ revised the final version of the paper. HW participated in the writing of the English paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Feng, M., Wu, X., Qiu, X. et al. Influence of Peat and Biochar on Gas Emissions and Microbial Metabolism During Co-composting of Chicken Manure and Maize Straw. Waste Biomass Valor 14, 197–208 (2023). https://doi.org/10.1007/s12649-022-01857-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01857-z