Abstract
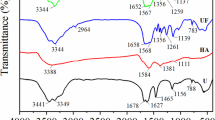
Based on weathered coal (WC), a new type of nitrogen-enriched weathered coal fertilizer (NCF) was synthesized herein using hydrogen peroxide and urea in a closed container. Inductively coupled plasma mass spectrometry, elemental analysis, scanning electron microscopy (SEM), Fourier-transform infrared (FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS), Contact angle, Zeta potential and X-ray diffraction (XRD) measurements were performed; the obtained results showed that NCF has potential slow-release nitrogen properties. Additionally, the FTIR and XPS measurements revealed that NCF has various oxygen-containing active functional groups along with nitrogen-containing groups. A soil leaching test and the release kinetic model fitting of total nitrogen showed that NCF released nitrogen more slowly and over a longer time than urea, with nitrogen still being released after 70 days. Further, a mechanism study was performed, which revealed that NCF has a dual release performance that is similar to that of biochar and “dissolved black nitrogen.” Moreover, the total nitrogen release behavior of NCF was dominated by the multi-effects of the coal carbon carrier, including electrostatic attraction, the unconventional formation of hydrogen bonds, and decomposition of the nitrogen-containing groups. Furthermore, the application of NCF in both soil types (Lou and Loessal soil) stimulated the germination rate of spinach seeds and improved the chloroplast pigment content as well as the nutritional quality of spinach leaves. These results suggest that abandoned weathered coal may be fully utilized in the mining area and that NCF may have promising potential in improving plant quality and sustainable agriculture applications.
Graphical Abstract















Similar content being viewed by others
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FTIR:
-
Fourier-transform infrared spectroscopy
- NCF:
-
Nitrogen-enriched weathered coal fertilizer
- SEM:
-
Scanning electron microscopy
- TG:
-
Thermogravimetric analysis
- TN:
-
Total nitrogen content
- WC:
-
Weathered coal
- WCA:
-
Water contact angle
- XPS:
-
X-ray photoelectron spectroscopy
- XRD:
-
X-ray diffraction
- CK:
-
The standard soil
References
Zhan, H., Yin, X., Huang, Y., Yuan, H., Wu, C.: Precursors evolving during rapid pyrolysis of lignocellulosic industrial biomass wastes-no x. Fuel 207, 438–448 (2017). https://doi.org/10.1016/j.fuel.2017.06.046
Zhang, S., Yuan, L., Li, W., Lin, Z., Li, Y., Hu, S., Zhao, B.: Characterization of pH-fractionated humic acids derived from Chinese weathered coal. Chemosphere 166, 334–342 (2017). https://doi.org/10.1016/j.chemosphere.2016.09.095
Carneiro, J.S.D.S., Lustosa Filho, J.F., Nardis, B.O., Ribeiro-Soares, J., Zinn, Y.L., Melo, L.C.A.: Carbon stability of engineered biochar-based phosphate fertilizers. ACS Sustain. Chem. Eng. 6, 14203–14212 (2018). https://doi.org/10.1021/acssuschemeng.8b02841
Wu, W., Yan, B., Sun, Y., Zhong, L., Lu, W., Chen, G.: Potential of yak dung-derived hydrochar as fertilizer: mechanism and model of controlled release of nitrogen. Sci. Total Environ. 781, 146665 (2021). https://doi.org/10.1016/j.scitotenv.2021.146665
Dinh, V.C., Hou, C.H., Dao, T.N.: O, N-doped porous biochar by air oxidation for enhancing heavy metal removal: the role of O, N functional groups. Chemosphere 293, 133622 (2022). https://doi.org/10.1016/j.chemosphere.2022.133622
Mian, M.M., Liu, G., Yousaf, B., Fu, B., Ullah, H., Ali, M.U., Abbas, Q., Mujtaba Munir, M.A., Ruijia, L.: Simultaneous functionalization and magnetization of biochar via NH3 ambiance pyrolysis for efficient removal of Cr (VI). Chemosphere 208, 712–721 (2018). https://doi.org/10.1016/j.chemosphere.2018.06.021
Wan, Z., Sun, Y., Tsang, D.C.W., Khan, E., Yip, A.C.K., Ng, Y.H., Rinklebe, J., Ok, Y.S.: Customised fabrication of nitrogen-doped biochar for environmental and energy applications. Chem. Eng. J. 401, 126136 (2020). https://doi.org/10.1016/j.cej.2020.126136
Wu, D., Song, W., Chen, L., Duan, X., Xia, Q., Fan, X., Li, Y., Zhang, F., Peng, W., Wang, S.: High-performance porous graphene from synergetic nitrogen doping and physical activation for advanced nonradical oxidation. J. Hazard. Mater. 381, 121010 (2020). https://doi.org/10.1016/j.jhazmat.2019.121010
Yu, W., Lian, F., Cui, G., Liu, Z.: N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 193, 8–16 (2018). https://doi.org/10.1016/j.chemosphere.2017.10.134
Zhou, Q., Jiang, X., Li, X., Jia, C.Q., Jiang, W.: Preparation of high-yield N-doped biochar from nitrogen-containing phosphate and its effective adsorption for toluene. RSC Adv. 8, 30171–30179 (2018). https://doi.org/10.1039/C8RA05714A
Zhu, S., Huang, X., Ma, F., Wang, L., Duan, X., Wang, S.: Catalytic removal of aqueous contaminants on n-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms. Environ. Sci. Technol. 52, 8649–8658 (2018). https://doi.org/10.1021/acs.est.8b01817
Bi, D., Huang, F., Jiang, M., He, Z., Lin, X.: Effect of pyrolysis conditions on environmentally persistent free radicals (EPFRs) in biochar from co-pyrolysis of urea and cellulose. Sci. Total Environ. 805, 150339 (2022). https://doi.org/10.1016/j.scitotenv.2021.150339
Hulicova-Jurcakova, D., Seredych, M., Lu, G.Q., Bandosz, T.J.: Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 19, 438–447 (2009). https://doi.org/10.1002/adfm.200801236
Liu, S.H., Huang, Y.Y.: Valorization of coffee grounds to biochar-derived adsorbents for CO2 adsorption. J. Clean. Prod. 175, 354–360 (2018). https://doi.org/10.1016/j.jclepro.2017.12.076
Zhang, F., Liu, T., Zhang, J., Cui, E., Yue, L., Jiang, R., Hou, G.: The potassium hydroxide-urea synergy in improving the capacitive energy-storage performance of agar-derived carbon aerogels. Carbon 147, 451–459 (2019). https://doi.org/10.1016/j.carbon.2019.03.011
Oh, W.D., Lisak, G., Webster, R.D., Liang, Y.N., Veksha, A., Giannis, A., Moo, J.G.S., Lim, J.W., Lim, T.T.: Insights into the thermolytic transformation of lignocellulosic biomass waste to redox-active carbocatalyst: durability of surface active sites. Appl. Catal. B 233, 120–129 (2018). https://doi.org/10.1016/j.apcatb.2018.03.106
Oh, W.D., Lok, L.W., Veksha, A., Giannis, A., Lim, T.T.: Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C3N4 under solar irradiation: performance and mechanistic studies. Chem. Eng. J. 333, 739–749 (2018). https://doi.org/10.1016/j.cej.2017.09.182
Wang, C., Wang, J., Wu, W., Qian, J., Song, S., Yue, Z.: Feasibility of activated carbon derived from anaerobic digester residues for supercapacitors. J. Power Sour. 412, 683–688 (2019). https://doi.org/10.1016/j.jpowsour.2018.11.092
Wang, H., Guo, W., Liu, B., Wu, Q., Luo, H., Zhao, Q., Si, Q., Sseguya, F., Ren, N.: Edge-nitrogenated biochar for efficient peroxydisulfate activation: an electron transfer mechanism. Water Res. 160, 405–414 (2019). https://doi.org/10.1016/j.watres.2019.05.059
Wang, Y., Chen, L., Chen, C., Xi, J., Cao, H., Duan, X., Xie, Y., Song, W., Wang, S.: Occurrence of both hydroxyl radical and surface oxidation pathways in N-doped layered nanocarbons for aqueous catalytic ozonation. Appl. Catal. B 254, 283–291 (2019). https://doi.org/10.1016/j.apcatb.2019.05.008
Zaeni, J.R.J., Lim, J.W., Wang, Z., Ding, D., Chua, Y.S., Ng, S.L., Oh, W.D.: In situ nitrogen functionalization of biochar via one-pot synthesis for catalytic peroxymonosulfate activation: characteristics and performance studies. Sep. Purif. Technol. 241, 116702 (2020). https://doi.org/10.1016/j.seppur.2020.116702
Fageria, N.K., Baligar, V.C.: Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 88, 97–185 (2005). https://doi.org/10.1016/S0065-2113(05)88004-6
Saha, B.K., Rose, M.T., Wong, V.N.L., Cavagnaro, T.R., Patti, A.F.: A slow release brown coal-urea fertiliser reduced gaseous N loss from soil and increased silver beet yield and N uptake. Sci. Total Environ. 649, 793–800 (2019). https://doi.org/10.1016/j.scitotenv.2018.08.145
Rose, M.T., Perkins, E.L., Saha, B.K., Tang, E.C.W., Cavagnaro, T.R., Jackson, W.R., Hapgood, K.P., Hoadley, A.F.A., Patti, A.F.: A slow release nitrogen fertiliser produced by simultaneous granulation and superheated steam drying of urea with brown coal. Chem. Biol. Technol. Agric. 3, 4842 (2016). https://doi.org/10.1186/s40538-016-0062-8
Saha, B.K., Rose, M.T., Wong, V.N.L., Cavagnaro, T.R., Patti, A.F.: Hybrid brown coal-urea fertiliser reduces nitrogen loss compared to urea alone. Sci. Total Environ. 601–602, 1496–1504 (2017). https://doi.org/10.1016/j.scitotenv.2017.05.270
Chen, S., Yang, M., Ba, C., Yu, S., Jiang, Y., Zou, H., Zhang, Y.: Preparation and characterization of slow-release fertilizer encapsulated by biochar-based waterborne copolymers. Sci. Total Environ. 615, 431–437 (2018). https://doi.org/10.1016/j.scitotenv.2017.09.209
González, M.E., Cea, M., Medina, J., González, A., Diez, M.C., Cartes, P., Monreal, C., Navia, R.: Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 505, 446–453 (2015). https://doi.org/10.1016/j.scitotenv.2014.10.014
Jiao, G.J., Peng, P., Sun, S.L., Geng, Z.C., She, D.: Amination of biorefinery technical lignin by Mannich reaction for preparing highly efficient nitrogen fertilizer. Int. J. Biol. Macromol. 127, 544–554 (2019). https://doi.org/10.1016/j.ijbiomac.2019.01.076
Yamamoto, C.F., Pereira, E.I., Mattoso, L.H.C., Matsunaka, T., Ribeiro, C.: Slow release fertilizers based on urea/urea-formaldehyde polymer nanocomposites. Chem. Eng. J. 287, 390–397 (2016). https://doi.org/10.1016/j.cej.2015.11.023
Fargašová, A., Molnárová, M.: Assessment of Cr and Ni phytotoxicity from cutlery-washing waste-waters using biomass and chlorophyll production tests on mustard Sinapis alba L. seedlings. Environ. Sci. Pollut. Res. 17, 187–194 (2010). https://doi.org/10.1007/s11356-009-0136-2
Zobiole, L.H.S., Bonini, E.A., de Oliveira, R.S., Kremer, R.J., Ferrarese-Filho, O.: Glyphosate affects lignin content and amino acid production in glyphosate-resistant soybean. Acta Physiol. Plant. 32(5), 831–837 (2010). https://doi.org/10.1007/s11738-010-0467-0
Cintya, H., Silalahi, J., Putra, E.D.L., Siburian, R.: The influence of fertilizer on nitrate, nitrite and vitamin C contents in vegetables. Orient. J. Chem. 34(5), 2614 (2018). https://doi.org/10.13005/ojc/340552
Razmjooei, F., Singh, K.P., Song, M.Y., Yu, J.S.: Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: a comprehensive study. Carbon 78, 257–267 (2014). https://doi.org/10.1016/j.carbon.2014.07.002
Merline, D.J., Vukusic, S., Abdala, A.A.: Melamine formaldehyde: curing studies and reaction mechanism. Polym. J. 45, 413–419 (2012). https://doi.org/10.1038/pj.2012.162
Lin, Z., Waller, G., Liu, Y., Liu, M., Wong, C.-P.: Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2, 884–888 (2012). https://doi.org/10.1002/aenm.201200038
Smith, M., Scudiero, L., Espinal, J., McEwen, J.-S., Garcia-Perez, M.: Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations. Carbon 110, 155–171 (2016). https://doi.org/10.1016/j.carbon.2016.09.012
Wan, L., Wang, J., Xie, L., Sun, Y., Li, K.: Nitrogen-enriched hierarchically porous carbons prepared from polybenzoxazine for high-performance supercapacitors. ACS Appl. Mater. Interfaces 6, 15583–15596 (2014). https://doi.org/10.1021/am504564q
Ma, D., Zhang, G., Areeprasert, C., Li, C., Shen, Y., Yoshikawa, K., Xu, G.: Characterization of NO emission in combustion of hydrothermally treated antibiotic mycelial residue. Chem. Eng. J. 284, 708–715 (2016). https://doi.org/10.1016/j.cej.2015.08.149
Pietrzak, R.: XPS study and physico-chemical properties of nitrogen-enriched microporous activated carbon from high volatile bituminous coal. Fuel 88, 1871–1877 (2009). https://doi.org/10.1016/j.fuel.2009.04.017
Zhan, H., Zhuang, X., Song, Y., Yin, X., Wu, C.: Insights into the evolution of fuel-N to NO x precursors during pyrolysis of N-rich nonlignocellulosic biomass. Appl. Energy 219, 20–33 (2018). https://doi.org/10.1016/j.apenergy.2018.03.015
Yan, J., Lei, Z., Li, Z., Wang, Z., Ren, S., Kang, S., Shui, H.: Molecular structure characterization of low-medium rank coals via XRD, solid state 13C NMR and FTIR spectroscopy. Fuel 268, 117038 (2020). https://doi.org/10.1016/j.fuel.2020.117038
Jiang, J., Yang, W., Cheng, Y., Liu, Z., Zhang, Q., Zhao, K.: Molecular structure characterization of middle-high rank coal via XRD, Raman and FTIR spectroscopy: implications for coalification. Fuel 239, 559–572 (2019). https://doi.org/10.1016/j.fuel.2018.11.057
Lu, L., Sahajwalla, V., Kong, C., Harris, D.: Quantitative X-ray diffraction analysis and its application to various coals. Carbon 39, 1821–1833 (2001). https://doi.org/10.1016/S0008-6223(00)00318-3
Lievens, C., Ci, D.H., Bai, Y., Ma, L., Zhang, R., Chen, J.Y., Gai, Q.Q., Long, Y.H., Guo, X.F.: A study of slow pyrolysis of one low rank coal via pyrolysis–GC/MS. Fuel Process. Technol. 116, 85–93 (2013). https://doi.org/10.1016/j.fuproc.2013.04.026
Kwok, D.Y., Neumann, A.W.: Contact angle measurement and contact angle interpretation. Adv. Coll. Interface Sci. 81, 167–249 (1999). https://doi.org/10.1016/S0001-8686(98)00087-6
Wang, S.T., Liu, K.S., Yao, X., Jiang, L.: Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem. Rev. 115, 8230–8293 (2015). https://doi.org/10.1021/cr400083y
Zhao, L., Cao, X., Zheng, W., Wang, Q., Yang, F.: Endogenous minerals have influences on surface electrochemistry and ion exchange properties of biochar. Chemosphere 136, 133–139 (2015). https://doi.org/10.1016/j.chemosphere.2015.04.053
Batista, E.M.C.C., Shultz, J., Matos, T.T.S., Fornari, M.R., Ferreira, T.M., Szpoganicz, B., Freitas, R.A., Mangrich, A.S.: Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 8, 10677 (2018). https://doi.org/10.1038/s41598-018-28794-z
Wang, H., Chen, X., Zhang, L., Li, Z., Fan, X., Sun, S.: Efficient production of lignin-based slow-release nitrogen fertilizer via microwave heating. Ind. Crops Prod. 166, 113481 (2021). https://doi.org/10.1016/j.indcrop.2021.113481
Liu, Z.X., Liu, Z.C., Zong, Z.M., Wei, X.Y., Wang, J., Lee, C.W.: GC/MS analysis of water-soluble products from the mild oxidation of Longkou brown coal with H2O2. Energy Fuels 17, 424–426 (2003). https://doi.org/10.1021/ef020071e
Xia, W.: The effect of heating on the wettability of lignite. Energy Sour. A 38, 3521–3526 (2016). https://doi.org/10.1080/15567036.2016.1194914
Chen, S., Tang, L., Tao, X., Chen, L., Yang, Z., Li, L.: Effect of oxidation processing on the surface properties and floatability of Meizhiyou long-flame coal. Fuel 210, 177–186 (2017). https://doi.org/10.1016/j.fuel.2017.08.053
Xu, M., Xing, Y., Gui, X., Cao, Y., Wang, D., Wang, L.: Effect of ultrasonic pretreatment on oxidized coal flotation. Energy Fuels 31, 14367–14373 (2017). https://doi.org/10.1021/acs.energyfuels.7b02115
Chen, W., Yang, H., Chen, Y., Chen, X., Fang, Y., Chen, H.: Biomass pyrolysis for nitrogen-containing liquid chemicals and nitrogen-doped carbon materials. J. Anal. Appl. Pyrol. 120, 186–193 (2016). https://doi.org/10.1016/j.jaap.2016.05.004
Li, Z., Liu, J., Xia, C., Li, F.: Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 3, 2440–2448 (2013). https://doi.org/10.1021/cs400506q
Peng, W., Zhang, H., Lü, F., Shao, L., He, P.: From food waste and its digestate to nitrogen self-doped char and methane-rich syngas: evolution of pyrolysis products during autogenic pressure carbonization. J. Hazard. Mater. 424, 127249 (2022). https://doi.org/10.1016/j.jhazmat.2021.127249
Maghsoodi, M.R., Najafi, N., Reyhanitabar, A., Oustan, S.: Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma 379, 114644 (2020). https://doi.org/10.1016/j.geoderma.2020.114644
Spokas, K.A., Novak, J.M., Venterea, R.T.: Biochar’s role as an alternative N-fertilizer: ammonia capture. Plant Soil 350, 35–42 (2011)
Taghizadeh-Toosi, A., Clough, T.J., Sherlock, R.R., Condron, L.M.: A wood based low-temperature biochar captures NH3-N generated from ruminant urine-N, retaining its bioavailability. Plant Soil 353, 73–84 (2011). https://doi.org/10.1007/s11104-011-1010-9
Mukherjee, A., Zimmerman, A.R., Harris, W.: Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 163, 247–255 (2011). https://doi.org/10.1016/j.geoderma.2011.04.021
Kammann, C.I., Schmidt, H.P., Messerschmidt, N., Linsel, S., Steffens, D., Müller, C., Koyro, H.-W., Conte, P., Joseph, S.: Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 5, 11080 (2015). https://doi.org/10.1038/srep11080
Zhang, H., Voroney, R.P., Price, G.W.: Effects of temperature and processing conditions on biochar chemical properties and their influence on soil C and N transformations. Soil Biol. Biochem. 83, 19–28 (2015). https://doi.org/10.1016/j.soilbio.2015.01.006
Zhu, D., Pignatello, J.J.: Characterization of Aromatic Compound Sorptive Interactions with Black Carbon (Charcoal) Assisted by Graphite as a Model. Environ. Sci. Technol. 39, 2033–2041 (2005). https://doi.org/10.1021/es0491376
De la Rosa, J.M., Knicker, H.: Bioavailability of N released from N-rich pyrogenic organic matter: an incubation study. Soil Biol. Biochem. 43, 2368–2373 (2011). https://doi.org/10.1016/j.soilbio.2011.08.008
Lian, F., Zhang, Y.K., Gu, S.G., Han, Y.R., Cao, X.S., Wang, Z.Y., Xing, B.S.: Photochemical transformation and catalytic activity of dissolved black nitrogen released from environmental black carbon. Environ. Sci. Technol. 55, 6476–6484 (2021). https://doi.org/10.1021/acs.est.1c00392
Liu, X., Liao, J., Song, H., Yang, Y., Guan, C., Zhang, Z.: A Biochar-based route for environmentally friendly controlled release of nitrogen: urea-loaded biochar and bentonite composite. Sci. Rep. 9, 9548 (2019). https://doi.org/10.1038/s41598-019-46065-3
Funding
This research was supported by the STS (Science and Technology Service Network Initiative) Program (KFJ-STS-QYZD-177), Innovation Chain of Key Industries-Social Development (2020ZDLSF06-09) and Innovation Chain of Key Industries- Agricultural Sector(2020ZDLNY01-08).
Author information
Authors and Affiliations
Contributions
XL, JZ and DS contributed to the conception and design of the study. XL, PZ, HJ, TJ, and HZ preformed the experiments and theoretical calculations, organized the database, and wrote the first draft of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article. II. Product Based: artificial fertilizers; IV. Process Based: waste-recycling;
Research Involving Human and Animal Rights
Uninvolved
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, X., She, D., Zhao, P. et al. Facile Synthesis a Potential Nitrogen-Enriched Weathered Coal Fertilizer: Excellent Slow-Release Performance and Improving Plant Quality. Waste Biomass Valor 13, 4685–4700 (2022). https://doi.org/10.1007/s12649-022-01778-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-01778-x