Abstract
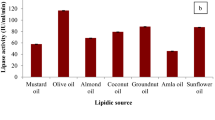
The present study investigates the biochemical characterization of an extracellular (phospho)lipase from a wood fungus Peziza sp. (medium optimization, inducer concentration and substrate specificity measurements). The strain was identified on the basis of ITS1/ITS4 primers. A 604 bp fragment was amplified by PCR and the obtained nucleotide sequence, showed 99% identity with the ITS region of isolates named as Peziza sp. Interestingly, Peziza sp. has both lipase and phospholipase activities with the same level which require both the presence of Ca2+ and bile salts. Our result shows that the lipase hydrolyzes preferably the olive oil at 45 °C, pH 8. Whereas, the phospholipase activity was detected on pure PC at 45 °C, pH 9. Lipid extraction from dry biomass using chloroform/methanol (2/1) and quantitative measurement using electron microscope showed that intracellular triglycerides content was significantly high and reaches 20.88%. Gas chromatography analysis shows a majority of C18:1 (76, 98%) and C18:2 (9, 33%). Whereas, saturated fatty acids ranging C16–C20 represent only 11.5% of total lipids composition.
Graphic Abstract
Lipid accumulation test in Pezizales sp under fluorescence microscope







Similar content being viewed by others
Abbreviations
- CPG:
-
Gas chromatography analysis
- PDA:
-
Potato Dextrose Agar
- PCR:
-
Polymerisation Chain Reaction
- PC:
-
Phosphatidyl choline
References
Sharma, R., Chisti, Y., Banerjee, U.C.: Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 19, 627–662 (2001)
Jaeger, K.E., Reetz, M.T.: Microbial lipases from versatile tools for biotechnology. Trends Biotechnol. 16, 396–403 (1998)
Rudd, E.A., Brockman, H.L.: Pancreatic carboxyl ester lipase (cholesterol esterase). In: Borgstrom, B., Brockman, H.L. (eds.) Lipases, pp. 185–204. Elsevier, Amsterdam (1984)
Verger, R.: ‘Interfacial activation’ of lipases: facts and artifacts. Trends Biotechnol. 15, 32–38 (1997)
Yong, S.K., Lim, B.H., Saleh, S., Tey, L.H.: Optimisation, purification and characterisation of extracellular lipase from Botryococcus sudeticus (UTEX 2629). J. Mol. Catal. B 126, 99–105 (2016)
Sharma, R., Chisti, Y., Banerjee, U.C.: Production, purification, characterization, and applications of lipases. Biotechnol. Adv. 19(8), 627–662 (2001)
Sharma, S., Kanwar, S.S.: Organic solvent tolerant lipases and applications. Sci. World J. 1155, 625258 (2014)
Ben Salah, R., Mosbah, H., Fendri, A., Gargouri, A., Gargouri, Y., Mejdoub, H.: Production of wild-type and peptide fusion cutinases by recombinantSaccharomyces cerevisiae MM01 strains. FEMS Microbiol. Lett. 260, 241–248 (2006)
Diaz, M.J.C., Rodríguez, J.A., Roussos, S., Cordova, J., Abousalham, A., Carrière, F., Bratti, J.: Lipase from the thermotolerant fungus Rhizopus homothallicus is more thermostable when produced using solid state fermentation than liquid fermentation procedures. Enzyme Microb. Technol. 39, 1042–1050 (2006)
Lima, V.M.G., Krieger, N., Mitchell, D.A., Fontana, J.D.: Activity and stability of acrude lipase from Penicillium aurantiogriseum in aqueous media and organic solvents. Biochem. Eng. J. 18, 65–71 (2004)
Derewenda, Z.S., Derewenda, U., Dodson, G.G.: The crystal and molecular structure of the Rhizomucor miehei triacylglyceride lipase at 1.9 A resolution. J. Mol. Biol. 227, 818–839 (1992)
Lafuente, F.R.: Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J. Mol. Catal. B 63, 17–22 (2010)
Kwon, M.A., Kim, H.S., Yang, T.H., Song, B.K., Song, J.K.: High-level expression andcharacterization of Fusarium solani cutinase in Pichia pastoris. Protein Expr. Purif. 68, 104–109 (2009)
Maia, M.M.D., Heasley, A., Camargo de Morais, M.M., Melo, E.H., Morais Jr., M.A., Ledingham, W.M., Lima Filho, J.L.: Effect of culture conditions on lipaseproduction by Fusarium solani in batch fermentation. Bioresour. Technol. 76, 23–27 (2001)
Jacob, J.S., Miller, K.R.: The effects of galactolipid depletion on the structure of a photosynthetic membrane. J. Cell Biol. 103, 1337–1347 (1986)
De Maria, L., Vind, J., Oxenbøll, K.M., Svendsen, A., Patkar, S.: Phospholipases and their industrial applications. Appl. Microbiol. Biotechnol. 74, 290–300 (2007)
Czabany, T., Athenstaedt, K., Daum, G.: Synthesis, storage and degradation of neutral lipids in yeast. Biochim. Biophys. Acta 1771(3), 299–309 (2007)
Hassan, M., Blanc, P.J., Pareilleux, A., Goma, G.: Selection of fatty acid auxotrophs from the oleaginous yeast Cryptococcus curvatus and production of coca butter equivalents in batch culture. Biotechnol Lett. 16(8), 819–824 (1994)
Zhu, L.Y., Zong, M.H., Wu, H.: Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour. Technol. 99(16), 7881–7885 (2008)
Demaison, L., Moreau, D.: Dietary n-3 polyunsaturated fatty acids and coronary heart disease-related mortality: a possible mechanism of action. Cell. Mol. Life Sci. 59, 463–477 (2002)
White, T.J., Bruns, T., Lee, S., Taylor, J.: Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J. (eds.) PCR Protocols: A Guide to Methods and Application, pp. 315–322. Academic Press, San Diego (1990)
Altschul, S.F., Madden, T.L., Schäffer, A.A., Zhang, J., Zhang, Z., Miller, W., Lipman, D.J.: Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25(17), 3389–3402 (1997)
Tiss, A., Carrière, F., Verger, R.: Effects of gum arabic on lipase interfacial binding and activity. Anal. Biochem. 294, 6–43 (2001)
Folch Br, J., Lees, M., Stanley, G.H.S.: A simple method for the isolation and purification of total lipidesfrom animal tissues. J. Biol. Chem. 226, 497 (1957)
Metcalfe, L.D., Schmitz, A.A.: The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 33(3), 363–364 (1961)
Kambourova, M., Kirilova, N., Mandeva, R., Derekova, A.: Purification and properties of thermostable lipase from a thermophilic Bacillus stearothermophilus MC7. J. Mol. Catal. B 22, 307–313 (2003)
Zaliha, R.N., Abd-Rahman, R., Leow, T.C., Salleh, A.B., Basri, M.: Geobacillus zalihae sp. nov., a thermophilic lipolytic bacterium isolated from palm oil mill effluent in Malaysia. BMC Microbiol. 7, 77 (2007)
Kumari, A., Mahapatra, P., Banerjee, R.: Statistical optimization of culture conditions by response surface methodology for synthesis of lipase with Enterobacter aerogenes. Braz. Arch. Biol. Technol. 52, 1349–1356 (2009)
Lopez, E., Deive, F.J., Longo, M.A., Sanroman, M.A.: Culture conditions and investigation of bioreactor configurations for lipase production by Rhizopus oryzae. Chem. Eng. Technol. 33, 1023–1028 (2010)
Lima, V.M.G., Krieger, N., Sarquis, M.I.M., Mitchell, D.A., Ramos, L.P., Fontana, J.D.: Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol. Biotechnol. 41, 105–110 (2003)
Treichel, H., de Oliveira, D., Mazutti, M.A., Di Luccio, M., Oliveira, J.V.: A review on microbial lipases production. Food Bioprocess Technol. 3, 182–196 (2010)
Wang, D., Xu, Y., Shan, T.: Effects of oils and oil-related substrates on the synthetic activity of membrane-bound lipase from Rhizopus chinensis and optimization of the lipase fermentation media. Biochem. Eng. J. 41, 30–37 (2008)
Abbas, H., Abel, H., Deyris, V., Comeau, L.: Isolation and characterization of an extracellular lipase from Mucor sp strain isolated from palm fruit. Enzym. Microb. Technol. 31, 968–975 (2002)
Ben Romdhane, I.B., Fendri, A., Gargouri, Y., Gargouri, A., Belghith, H.: A novel thermoactive and alkaline lipase from Talaromyces thermophilus fungus for use in laundry detergents. Biochem. Eng. J. 53, 112–120 (2010)
Wu, X.Y., Jäskeläinen, S., Linko, W.Y.: Purification and partial characterization of Rhizomucor miehei lipase for ester synthesis. Appl. Biochem. Biotechnol. 59, 145–158 (1996)
Sugihara, A., Shimada, Y., Tominaga, Y.: Purification and characterization of Aspergillus niger lipase. Agric. Biol. Chem. 52, 1591–1592 (1988)
Chopra, A.K., Chander, H., Singh, J.: Lipolytic activity of Syncephalastrum racemosum. J. Dairy Sci. 65, 1890–1894 (1982)
Simons, J.-W.F.A., Hendrik, A., Ruud, C.C., Niek, D., Friedrich, G., Arend, J.S.: The lipase from Staphylococcus aureus. Eur. J. Biochem. 242, 760–769 (1996)
Gray, C.J.: Stabilization of enzymes with soluble additives. In: Gupta, M.N. (ed.) Thermostability of Enzymes in Stabilization of Enzymes with Soluble Additives, pp. 124–143. Springer, New Delhi (1993)
Aoki, J., Inoue, A., Makide, K., Saiki, N., Arai, H.: Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie 89, 197–204 (2007)
Jallouli, R., Khrouf, F., Fendri, A., Mechichi, T., Gargouri, Y., Bezzine, S.: Purification and biochemical characterization of a novel alkaline (phospho)lipase from a newly isolated Fusarium solani strain. Appl. Biochem. Biotechnol. 168, 2330–2343 (2012). https://doi.org/10.1007/s12010-012-9940-0
Knothe, G.: Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2, 759–766 (2009)
Acknowledgements
This work is a part of a doctoral thesis by Ameni KTATA. Whose research was supported financially by “Ministère de l’enseignement supérieur, de la recherche scientifique et de la technologie-Tunisia” through a grant to “Laboratoire de Biochimie et Génie Enzymatique des Lipases-ENIS”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ktata, A., Bezzine, S., Sayari, A. et al. Newly Isolated Lipolytic and Oleaginous Fungal Strain, Production, Optimization and Biochemical Characterization of the Extracellular (phospho)lipase. Waste Biomass Valor 11, 6677–6687 (2020). https://doi.org/10.1007/s12649-019-00907-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00907-3