Abstract
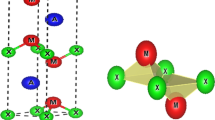
First principle calculations of structural, elastic, thermal and electronic properties of M2X (M = Sr, Ba and X = Si, Ge, Sn) compounds in the anti-fluorite type structure are performed within the framework of density functional theory. The lattice constant, bulk modulus, derivative of bulk modulus and ground state energy are calculated. The calculated elastic properties reveal that Sr2Si, Sr2Ge, Ba2Si and Ba2Ge are classified as brittle, while Sr2Sn and Ba2Sn show ductile nature. It is noted that these compounds are elastically stable and the Debye temperature value decreases from Sr to Ba and from top to bottom in group IV-A of periodic table. From electronic charge density plot in the (110) crystallographic plane it is observed that in Sr2X, Sr shows ionic bond nature with X, while in Ba2X, Ba forms partially ionic and partially covalent bond with X. The density of states and the electronic band structures are also presented. We have found that these compounds possess narrow and direct band gaps. The values of the energy gaps obtained by using the modified Becke–Johnson approach are better than the values obtained from the generalized gradient approximation.





Similar content being viewed by others
References
J Hu, A Kato, T Sadoh, Y Maeda, K N Galkin, T V Turchin and H Tatsuoka Int. J. Mod. Phys. B. 24 3693 (2010)
B I Sharma, J Maibam, R S Paul, R K Thapa and R K B Singh Indian J. Phys. 84 671 (2010)
S Brutti, D Nguyen-Manh and D G Pettifor J. Alloys Compd. 457 29 (2008)
Y Sun, X Q Chen, D Li, C Franchini, S Yunoki, Y Li and Z Fang Phys. Rev. B 84 165127 (2011)
Y Imai, A Watanabe and M Mukaida J. Alloy. Compd. 358 257 (2003)
B. Arnaud and M. Alouani Phys. Rev. B 64 033202 (2001)
A Vantomme et al. Appl. Phys. Lett. 70 1086 (1997)
F Kalarasse and B Bennecer J. Phys. Chem. Solids 69 1775 (2008)
D M Wood and A Zunger Phys. Rev. B 34 4105 (1986)
J Tejeda and M Cardona Phys. Rev. B 14 2559 (1976)
P Hohenberg and W Kohn Phys. Rev. B 136 864 (1964)
D D Koelling and B N Harmon J. Phys. C:Solid State Phys. 10 3107 (1977)
P Blaha, K Schwarz, G K H Madsen, D Kvasnicka and J Luitz, Wien2k, An Augmented Plane Wave Local orbitals program for calculating crystal properties Karlheinz Schwarz, Techn. Universitat, Wien, Austria, ISBN 3-9501031-1-2(2001)
J P Perdew, S Burke and M Ernzerhof Phys. Rev. Lett. 77 3865 (1996)
F Tran and P Blaha Phy. Rev. Lett. 102 226401 (2009)
G Murtaza and I Ahmad Phys. B. 406 3222 (2011)
H Ud Din and A H Reshak Comput. Mater. Sci. 83 474 (2014)
A H Reshak, S Azam, ZA Alahmed and J Chyský J. Magn. Magn. Mater. 351 98 (2014)
B Amin, S Nazir and U Schwingenschlogl Sci. Rep. 3 1705 (2013)
S A Khan and A H Reshak Int. J. Electrochem. Sci. 8 9459 (2013)
A H Reshak, H Kamarudin and S Auluk J. Phys. Chem. B. 116 4677 (2012)
R Ali, S Mohammad, H Ullah, S A Khan, H Ud Din, M Khan and N U Khan Phys. B 410 93 (2013)
F Birch J. Geophys. Res. 83 1257 (1978)
M J Mehl, B M Barry and D A Papaconstantopoulos Intermetallic Compounds: Principle and Practice, Volume 1: Principles (London: John Wiley and Sons) ed. J H Westbrook and R L Fleischeir p 195 (1995)
B Mayer, H Anton, E Bott, M Methfessel, J Sticht and P C Schmidt Intermetallics 11 23 (2003)
S F Pugh Philos. Mag. 45 823 (1954)
Z Sun, S Li, R Ahuja and J M Schneide Solid. State Commun. 129 589 (2004)
P Wachter, M Filzmoser and J Rebizant Phys. B. 293 199 (2001)
OL Anderson J. Phys. Chem. Solids 24 909 (1963)
Acknowledgments
The work was supported by CENTEM project, reg. no. CZ.1.05/2.1.00/03.0088, co-funded by the ERDF as part of the Ministry of Education, Youth and Sports OP RDI program. Computational resources were provided by MetaCentrum (LM2010005) and CERIT-SC (CZ.1.05/3.2.00/08.0144) infrastructures. For the author (Z. A. Alahmed) the project was supported by the Research Center, College of Science, King Saud University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ud Din, H., Reshak, A.H., Murtaza, G. et al. Structural, elastic, thermal and electronic properties of M2X (M = Sr, Ba and X = Si, Ge, Sn) compounds in anti-fluorite structure: first principle calculations. Indian J Phys 89, 369–375 (2015). https://doi.org/10.1007/s12648-014-0585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12648-014-0585-4