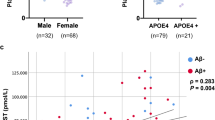
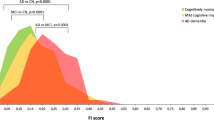
Abstract
Relationship between serum calcium and Alzheimer’s disease (AD) remains unclear. The aim of this study is to test whether serum calcium is associated with other AD-associated biomarkers and could predict clinical progression in nondemented elders. This was a longitudinal population-based study. The sample was derived from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort, which included 1224 nondemented elders: 413 cognitively normal (CN) and 811 mild cognition impairment (MCI). Associations were investigated between serum calcium and longitudinal changes in Aβ/tau pathologic features, brain structure, cognitive function, and disease progression. Serum calcium concentrations increased with disease severity. Serum calcium predicted longitudinal cognitive decline and conversion from nondemented status to AD dementia (adjusted HR = 1.41, 95% CI 1.13–1.76). Furthermore, serum calcium levels were negatively correlated with CSF-Aβ42 (β = − 0.558, P = 0.008), FDG-PET (β = − 0.292, P < 0.001), whole brain volume (β = − 0.148, P = 0.001), and middle temporal volume (β = − 0.216, P = 0.042). Similar results were obtained in CN and MCI groups. Higher serum calcium status (even if not hypercalcemia) may increase the risk of AD in elders. Serum calcium is a useful biomarker in predicting clinical progression in nondemented elders. More researches are needed in the future to explore the underlying mechanism.



Similar content being viewed by others
References
Apostolova LG et al (2010) 3D PIB and CSF biomarker associations with hippocampal atrophy in ADNI subjects. Neurobiol Aging 31:1284–1303. https://doi.org/10.1016/j.neurobiolaging.2010.05.003
Bennett DA et al (2004) Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol 61:378–384. https://doi.org/10.1001/archneur.61.3.378
Betzer C et al (2018) Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation EMBO reports 19. https://doi.org/10.15252/embr.201744617
Bolland MJ et al (2010) Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ (Clinical Research Ed) 341:c3691. https://doi.org/10.1136/bmj.c3691
Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011) Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ (Clinical Research Ed) 342:d2040. https://doi.org/10.1136/bmj.d2040
Bushinsky DA, Monk RD (1998) Electrolyte quintet: Calcium Lancet (London, England) 352:306–311. https://doi.org/10.1016/s0140-6736(97)12331-5
Bussiere R et al (2019) Upregulation of the sarco-endoplasmic reticulum calcium ATPase 1 truncated isoform plays a pathogenic role in Alzheimer’s disease Cells 8. https://doi.org/10.3390/cells8121539
Crane PK et al (2012) Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav 6:502–516. https://doi.org/10.1007/s11682-012-9186-z
Eichler T et al (2014) Rates of formal diagnosis in people screened positive for dementia in primary care: results of the DelpHi-Trial. J Alzheimers Dis 42:451–458. https://doi.org/10.3233/jad-140354
Gibbons LE et al (2012) A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain Imaging Behav 6:517–527. https://doi.org/10.1007/s11682-012-9176-1
Hendrix JA et al (2015) The worldwide Alzheimer’s Disease Neuroimaging Initiative: an update. Alzheimer’s Dement 11:850–859. https://doi.org/10.1016/j.jalz.2015.05.008
Hotchkiss RS, Strasser A, McDunn JE, Swanson PE (2009) Cell death. N Engl J Med 361:1570–1583. https://doi.org/10.1056/NEJMra0901217
Jagust WJ et al (2009) Relationships between biomarkers in aging and dementia. Neurology 73:1193–1199. https://doi.org/10.1212/WNL.0b013e3181bc010c
Jessen F et al (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10:844–852. https://doi.org/10.1016/j.jalz.2014.01.001
Joborn C et al (1991) Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism Psychoneuroendocrinology 16:311–322. https://doi.org/10.1016/0306-4530(91)90017-n
Kawahara M, Kuroda Y (2000) Molecular mechanism of neurodegeneration induced by Alzheimer’s beta-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull 53:389–397. https://doi.org/10.1016/s0361-9230(00)00370-1
Kern J et al (2016) Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 87:1674–1680. https://doi.org/10.1212/wnl.0000000000003111
Lacampagne A et al (2017) Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol 134:749–767. https://doi.org/10.1007/s00401-017-1733-7
McKhann G et al (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34:939–944. https://doi.org/10.1212/wnl.34.7.939
Ogihara T, Miya K, Morimoto S (1990) Possible participation of calcium-regulating factors in senile dementia in elderly female subjects. Gerontology 36(Suppl 1):25–30. https://doi.org/10.1159/000213230
Payne RB, Little AJ, Williams RB, Milner JR (1973) Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4:643–646. https://doi.org/10.1136/bmj.4.5893.643
Petersen RC et al (2010) Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 74:201–209. https://doi.org/10.1212/WNL.0b013e3181cb3e25
Sato K et al (2019) Lower serum calcium as a potentially associated factor for conversion of mild cognitive impairment to early Alzheimer’s disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative. J Alzheimers Dis 68:777–788. https://doi.org/10.3233/jad-181115
Schram MT et al (2007) Serum calcium and cognitive function in old age. J Am Geriatr Soc 55:1786–1792. https://doi.org/10.1111/j.1532-5415.2007.01418.x
Shaw LM et al (2009) Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65:403–413. https://doi.org/10.1002/ana.21610
Sperling RA et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Subhash MN et al (1991) Calcium and phosphorus levels in serum and CSF in dementia. Neurobiol Aging 12:267–269. https://doi.org/10.1016/0197-4580(91)90001-z
Tilvis RS et al (2004) Predictors of cognitive decline and mortality of aged people over a 10-year period . J.Gerontol A Biol Sci Med Sci 59:268–274. https://doi.org/10.1093/gerona/59.3.m268
Toescu EC, Verkhratsky A, Landfield PW (2004) Ca2+ regulation and gene expression in normal brain aging Trends. Neurosci 27:614–620. https://doi.org/10.1016/j.tins.2004.07.010
Walker MD, Silverberg SJ (2008) Cardiovascular aspects of primary hyperparathyroidism. J Endocrinol Invest 31:925–931. https://doi.org/10.1007/bf03346443
Yarlagadda A, Kaushik S, Clayton AH (2007) Blood brain barrier: the role of calcium homeostasis Psychiatry (Edgmont (Pa : Township)) 4:55–59.
Yurko R, G’Sell M, Roeder K, Devlin B (2020) A selective inference approach for false discovery rate control using multiomics covariates yields insights into disease risk. Proc Natl Acad Sci USA 117:15028–15035. https://doi.org/10.1073/pnas.1918862117
Zhang D et al (2011) Multimodal classification of Alzheimer’s disease and mild cognitive impairment. NeuroImage 55:856–867. https://doi.org/10.1016/j.neuroimage.2011.01.008
Zhen D et al (2015) High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone 71:1–6. https://doi.org/10.1016/j.bone.2014.09.024
Funding
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This study was supported by grants from the National Natural Science Foundation of China (91849126, 81571245, and 81771148), the National Key R&D Program of China (2018YFC1314700), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, and the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, Fudan University.
Author information
Authors and Affiliations
Contributions
Ling-Zhi Ma, Zi-Xuan Wang, Qiang Dong, Lan Tan, and Jin-Tai Yu planed the work and drafted the manuscript; Ling-Zhi Ma, Zi-Xuan Wang, Zuo-Teng Wang, and Xiao-He Hou helped in the manuscript preparation; Ling-Zhi Ma, Zi-Xuan Wang, Xue-Ning Shen, and Ya-Nan Ou designed and has drawn the figures and pathway; Lan Tan and Jin-Tai Yu guided throughout the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, LZ., Wang, ZX., Wang, ZT. et al. Serum Calcium Predicts Cognitive Decline and Clinical Progression of Alzheimer’s Disease. Neurotox Res 39, 609–617 (2021). https://doi.org/10.1007/s12640-020-00312-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-020-00312-y