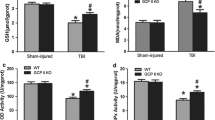
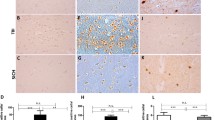
Abstract
Following acute neuronal lesions, metabolic imbalance occurs, the rate of glycolysis increases, and methylglyoxal (MGO) forms, finally leading to metabolic dysfunction and inflammation. The glyoxalase system is the main detoxification system for MGO and is impaired following excitotoxicity and stroke. However, it is not known yet whether alterations of the glyoxalase system are also characteristic for other neuronal damage models. Neuronal damage was induced in organotypic hippocampal slice cultures by transection of perforant pathway (PPT; 5 min to 72 h) and N-methyl-d-aspartate (NMDA; 50 μM for 4 h) or in vivo after controlled cortical impact (CCI) injury (2 h to 14 days). Temporal and spatial changes of glyoxalase I (GLO1) were investigated by Western blot analyses and immunohistochemistry. In immunoblot, the GLO1 protein content was not significantly affected by PPT at all investigated time points. As described previously, NMDA treatment led to a GLO1 increase 24 and 48 h after the lesion, whereas PPT increased GLO1 immunoreactivity within neurons only at 48 h postinjury. Immunohistochemistry of brain tissue subjected to CCI unveiled positive GLO1 immunoreactivity in neurons and astrocytes at 1 and 3 days after injury. Two hours and 14 days after CCI, no GLO1 immunoreactivity was observed. GLO1 protein content changes are associated with excitotoxicity but seemingly not to fiber transection. Cell-specific changes in GLO1 immunoreactivity after different in vitro and in vivo lesion types might be a common phenomenon in the aftermath of neuronal lesions.




Similar content being viewed by others
References
Ahmed N, Battah S, Karachalias N, Babaei-Jadidi R, Horanyi M, Baroti K, Hollan S, Thornalley PJ (2003) Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim Biophys Acta 1639(2):121–132
Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O (2012) The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma 29(12):2181–2191. https://doi.org/10.1089/neu.2011.2067
Almeida A, Moncada S, Bolanos JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6(1):45–51
Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagepetersen HS (2009) Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem 109(Suppl 1):87–93
Bechmann I, Nitsch R (1997) Astrocytes and microglial cells incorporate degenerating fibers following entorhinal lesion: a light, confocal, and electron microscopical study using a phagocytosis-dependent labeling technique. Glia 20(2):145–154
Belanger M, Yang J, Petit J-M, Laroche T, Magistretti PJ, Allaman I (2011) Role of the glyoxalase system in astrocyte-mediated neuroprotection. J Neurosci 31(50):18338–18352
Birkenmeier G, Stegemann C, Hoffmann R, Günther R, Huse K, Birkemeyer C (2010) Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS One 5(4):e10399
Bolanos JP, Almeida A, Moncada S (2010) Glycolysis: a bioenergetic or a survival pathway? Trends Biochem Sci 35(3):145–149
Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA (2008) A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28(1):264–278
Chu JMT, Lee DKM, Wong DPK, Wong GTC, Yue KKM (2016) Methylglyoxal-induced neuroinflammatory response in in vitro astrocytic cultures and hippocampus of experimental animals. Metab Brain Dis 31(5):1055–1064
de Arriba SG, Krugel U, Regenthal R, Vissiennon Z, Verdaguer E, Lewerenz A, Garcia-Jorda E, Pallas M, Camins A, Munch G, Nieber K, Allgaier C (2006) Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic Biol Med 40(5):779–790
Deller T, Del Turco D, Rappert A, Bechmann I (2007) Structural reorganization of the dentate gyrus following entorhinal denervation: species differences between rat and mouse. Prog Brain Res 163:501–528
Derouiche A (2000) Topical correlation of increased hippocampal glutamine synthetase immunoreactivity and glutamatergic terminal fields after entorhinal cortex lesion. Glia 29(4):386–391
Di Loreto S, Caracciolo V, Colafarina S, Sebastiani P, Gasbarri A, Amicarelli F (2004) Methylglyoxal induces oxidative stress-dependent cell injury and up-regulation of interleukin-1beta and nerve growth factor in cultured hippocampal neuronal cells. Brain Res 1006(2):157–167
Di Loreto S, Zimmitti V, Sebastiani P, Cervelli C, Falone S, Amicarelli F (2008) Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int J Biochem Cell Biol 40(2):245–257
Distler MG, Plant LD, Sokoloff G, Hawk AJ, Aneas I, Wuenschell GE, Termini J, Meredith SC, Nobrega MA, Palmer AA (2012) Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J Clin Invest 122(6):2306–2315
Doll DN, Barr TL, Simpkins JW (2014) Cytokines: their role in stroke and potential use as biomarkers and therapeutic targets. Aging Dis 5(5):294–306
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62(6):649–671
Dusart I, Schwab ME (1994) Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci 6(5):712–724
Ebrahimi F, Koch M, Pieroh P, Ghadban C, Hobusch C, Bechmann I, Dehghani F (2012) Time dependent neuroprotection of mycophenolate mofetil: effects on temporal dynamics in glial proliferation, apoptosis, and scar formation. J Neuroinflammation 9:89
Greenberg CS, Birckbichler PJ, Rice RH (1991) Transglutaminases: multifunctional cross-linking enzymes that stabilize tissues. FASEB J 5(15):3071–3077
Hailer NP, Grampp A, Nitsch R (1999) Proliferation of microglia and astrocytes in the dentate gyrus following entorhinal cortex lesion: a quantitative bromodeoxyuridine-labelling study. Eur J Neurosci 11(9):3359–3364
Ientile R, Caccamo D, Macaione V, Torre V, Macaione S (2002) NMDA-evoked excitotoxicity increases tissue transglutaminase in cerebellar granule cells. Neuroscience 115(3):723–729
Kallendrusch S, Hobusch C, Ehrlich A, Ziebell S, Ueda N, Geisslinger G, Koch M, Dehghani F (2012) Site-specific and time-dependent activation of the endocannabinoid system after transection of long-range projections. PLoS One 7(3):e33537
Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW (2004) Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science 305(5680):99–103
Kikuchi S, Shinpo K, Moriwaka F, Makita Z, Miyata T, Tashiro K (1999) Neurotoxicity of methylglyoxal and 3-deoxyglucosone on cultured cortical neurons: synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases. J Neurosci Res 57(2):280–289
Kovac AD, Kwidzinski E, Heimrich B, Bittigau P, Deller T, Nitsch R, Bechmann I (2004) Entorhinal cortex lesion in the mouse induces transsynaptic death of perforant path target neurons. Brain Pathol 14(3):249–257
Kuhla B, Luth H-J, Haferburg D, Weick M, Reichenbach A, Arendt T, Munch G (2006) Pathological effects of glyoxalase I inhibition in SH-SY5Y neuroblastoma cells. J Neurosci Res 83(8):1591–1600
Kuhla B, Boeck K, Schmidt A, Ogunlade V, Arendt T, Munch G, Luth H-J (2007) Age- and stage-dependent glyoxalase I expression and its activity in normal and Alzheimer's disease brains. Neurobiol Aging 28(1):29–41
Kumagai T, Nangaku M, Kojima I, Nagai R, Ingelfinger JR, Miyata T, Fujita T, Inagi R (2009) Glyoxalase I overexpression ameliorates renal ischemia-reperfusion injury in rats. Am J Physiol Renal Physiol 296(4):912–921
Kurz A, Rabbani N, Walter M, Bonin M, Thornalley P, Auburger G, Gispert S (2011) Alpha-synuclein deficiency leads to increased glyoxalase I expression and glycation stress. Cell Mol Life Sci 68(4):721–733
Lee D-Y, Chang G-D (2014) Methylglyoxal in cells elicits a negative feedback loop entailing transglutaminase 2 and glyoxalase 1. Redox Biol 2:196–205
Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH-C, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M (2007) The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 27(45):12255–12266
Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A (2008) Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J 27(20):2736–2745
Mastrocola R, Nigro D, Cento AS, Chiazza F, Collino M, Aragno M (2016) High-fructose intake as risk factor for neurodegeneration: key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91(22):10625–10629
Phillips SA, Thornalley PJ (1993) The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur J Biochem 212(1):101–105
Pieroh P, Koch M, Wagner D-C, Boltze J, Ehrlich A, Ghadban C, Hobusch C, Birkenmeier G, Dehghani F (2014a) Temporal dynamics of glyoxalase 1 in secondary neuronal injury. PLoS One 9(2):e87364
Pieroh P, Birkenmeier G, Dehghani F (2014b) The temporal and spatial dynamics of glyoxalase I following excitoxicity and brain ischaemia. Biochem Soc Trans 42(2):534–537
Richard JP (1993) Mechanism for the formation of methylglyoxal from triosephosphates. Biochem Soc Trans 21(2):549–553
Rodriguez-Rodriguez P, Fernandez E, Almeida A, Bolanos JP (2012) Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell Death Differ 19(10):1582–1589
Selkoe DJ, Abraham C, Ihara Y (1982) Brain transglutaminase: in vitro crosslinking of human neurofilament proteins into insoluble polymers. Proc Natl Acad Sci U S A 79(19):6070–6074
Sellin S, Mannervik B (1983) Reversal of the reaction catalyzed by glyoxalase I. Calculation of the equilibrium constant for the enzymatic reaction. J Biol Chem 258(14):8872–8875
Shin MJ, Kim DW, Lee YP, Ahn EH, Jo HS, Kim D-S, Kwon O-S, Kang T-C, Cho Y-J, Park J, Eum WS, Choi SY (2014) Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic Biol Med 67:195–210
Stoppini L, Buchs PA, Muller D (1991) A simple method for organotypic cultures of nervous tissue. J Neurosci Methods 37(2):173–182
Tajes M, Guivernau B, Ramos-Fernandez E, Bosch-Morato M, Palomer E, Guix FX, Munoz FJ (2013) The pathophysiology of triose phosphate isomerase dysfunction in Alzheimer's disease. Histol Histopathol 28(1):43–51
Tator CH (1995) Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 5(4):407–413
Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Asp Med 14(4):287–371
Author information
Authors and Affiliations
Contributions
P.P., G.B., and F.D. conceived and designed the experiments; P.P., B.A., M.D.-N., A.E, C.G., and C.H. performed the experiments; P.P., D-C.W., G.B., and F.D. analyzed the data; B.A., M.D.-N., D-C.W., J.B., G.B., and F.D contributed reagents/materials/analysis tools; P.P., B.A., D-C.W., G.B., and F.D wrote the paper.
Corresponding author
Ethics declarations
All animal experiments were performed in accordance with the Policy on Ethics and the Policy on the Use of Animals in Neuroscience Research as indicated in the directive 2010/63/EU of the European Parliament and of the Council of the European Union on the protection of animals used for scientific purposes. The local authorities approved controlled cortical impact experiments for care and use of laboratory animals (State of Rhineland-Palatinate: 1.5 177-07/051-31), and experiments were performed under all efforts to minimize suffering.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 15 kb).
Rights and permissions
About this article
Cite this article
Pieroh, P., Wagner, DC., Alessandri, B. et al. Comparative Examination of Temporal Glyoxalase 1 Variations Following Perforant Pathway Transection, Excitotoxicity, and Controlled Cortical Impact Injury. Neurotox Res 33, 412–421 (2018). https://doi.org/10.1007/s12640-017-9808-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-017-9808-8