Abstract
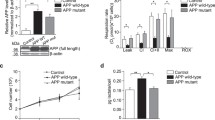
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder that is thought to be caused in part by the age-related accumulation of amyloid-β (Aβ) in the brain. Recent findings have revealed that nitric oxide (NO) modulates the processing of amyloid-β precursor protein (APP) and alters Aβ production; however, the previously presented data are contradictory and the underlying molecular mechanisms are still incomplete. Here, using human SH-SY5Y neuroblastoma cells stably transfected with wild-type APPwt695, we found that NO, derived from NO donor sodium nitroprusside (SNP), bi-directionally modulates APP processing in vitro. The data from ELISA and Western blot (WB) tests indicated that SNP at lower concentrations (0.01 and 0.1 μM) inhibits BACE1 expression, thus consequently suppresses APP β-cleavage and decreases Aβ production. In contrast, SNP at higher concentrations (10 and 20 μM) biases the APP processing toward the amyloidogenic pathway as evidenced by an increased BACE1 but a decreased ADAM10 expression, together with an elevated Aβ secretion. This bi-directional modulating activity of SNP on APP processing was completely blocked by specific NO scavenger c-PTIO, indicating NO-dependent mechanisms. Moreover, the anti-amyloidogenic activity of SNP is sGC/cGMP/PKG-dependent as evidenced by its reversal by sGC/PKG inhibitions, whereas the amyloidogenic activity of SNP is peroxynitrite-related and can be reversed by peroxynitrite scavenger uric acid. In summary, these present findings predict a double-edged role of NO in APP processing in vitro. Low (physiological) levels of NO inhibit the amyloidogenic processing of APP, whereas extra-high (pathological) concentrations of NO favor the amyloidogenic pathway of APP processing. This preliminary study may provide further evidence to clarify the molecular roles of NO and NO-related signaling in AD and supply potential molecular targets for AD treatment.








Similar content being viewed by others
References
Akama KT, Albanese C, Pestell RG, Van Eldik LJ (1998) Amyloid beta-peptide stimulates nitric oxide production in astrocytes through an NFκB-dependent mechanism. Proc Natl Acad Sci USA 95:5795–5800
Araki W, Kitaguchi N, Tokushima Y, Ishii K, Aratake H, Shimohama S, Nakamura S, Kimura J (1991) Trophic effect of β-amyloid precursor protein on cerebral cortical neurons in culture. Biochem Biophys Res Commun 181:265–271
Austin SA, Santhanam AV, Katusic ZS (2010) Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 107:1498–1502
Austin SA, d’Uscio LV, Katusic ZS (2013a) Supplementation of nitric oxide attenuates AβPP and BACE1 protein in cerebral microcirculation of eNOS-deficient mice. J Alzheimers Dis 33:29–33
Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS (2013b) Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J Neurochem 127:691–700
Bell KF, Zheng L, Fahrenholz F, Cuello AC (2008) ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol Aging 29:554–565
Borlikova GG, Trejo M, Mably AJ, Mc Donald JM, Sala Frigerio C, Regan CM, Murphy KJ, Masliah E, Walsh DM (2013) Alzheimer brain-derived amyloid β-protein impairs synaptic remodeling and memory consolidation. Neurobiol Aging 34:1315–1327
Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A (2012) Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 109:8740–8745
Chasseigneaux S, Allinquant B (2012) Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J Neurochem 120(Suppl 1):99–108
Chiasson VL, Munshi N, Chatterjee P, Young KJ, Mitchell BM (2011) Pin1 deficiency causes endothelial dysfunction and hypertension. Hypertension 58:431–438
Choi HS, Kim JW, Cha YN, Kim C (2006) A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem 27:31–44
Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH (2005) Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci 8:79–84
de la Torre JC, Pappas BA, Prevot V, Emmerling MR, Mantione K, Fortin T, Watson MD, Stefano GB (2003) Hippocampal nitric oxide upregulation precedes memory loss and Aβ1-40 accumulation after chronic brain hypoperfusion in rats. Neurol Res 25:635–641
Dewachter I, Van Leuven F (2002) Secretases as targets for the treatment of Alzheimer’s disease: the prospects. Lancet Neurol 1:409–416
Driver JA, Zhou XZ, Lu KP (2014) Regulation of protein conformation by Pin1 offers novel disease mechanisms and therapeutic approaches in Alzheimer’s disease. Discov Med 17:93–99
Driver JA, Zhou XZ, Lu KP (2015) Pin1 dysregulation helps to explain the inverse association between cancer and Alzheimer’s disease. Biochim Biophys Acta 1850:2069–2076
Esler WP, Wolfe MS (2001) A portrait of Alzheimer secretases—new features and familiar faces. Science 293:1449–1454
Furukawa K, Sopher BL, Rydel RE, Begley JG, Pham DG, Martin GM, Fox M, Mattson MP (1996) Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem 67:1882–1896
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314:777–781
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356
Heales SJ, Bolaños JP, Stewart VC, Brookes PS, Land JM, Clark JB (1999) Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1410:215–228
Jamsa A, Belda O, Edlund M, Lindstrom E (2011) BACE-1 inhibition prevents the gamma-secretase inhibitor evoked Abeta rise in human neuroblastoma SH-SY5Y cells. J Biomed Sci 18:76
Jin M, Shepardson N, Yang T, Chen G, Walsh D, Selkoe DJ (2011) Soluble amyloid β-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA 108:5819–5824
Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, Müller-Spahn F, Haass C, Czech C, Pradier L, Müller WE, Eckert A (2004) Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem 279:50310–50320
Kwak YD, Wang R, Li JJ, Zhang YW, Xu H, Liao FF (2011) Differential regulation of BACE1 expression by oxidative and nitrosative signals. Mol Neurodegener 6:17
Law A, Gauthier S, Quirion R (2001) Say NO to Alzheimer’s disease: the putative links between nitric oxide and dementia of the Alzheimer’s type. Brain Res Rev 35:73–96
Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH (2006) A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440:352–357
Li S, Wang W, Wang C, Tang YY (2010) Possible involvement of NO/NOS signaling in hippocampal amyloid-beta production induced by transient focal cerebral ischemia in aged rats. Neurosci Lett 470:106–110
Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the β-amyloid precursor protein. Neuron 10:243–254
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Pak T, Cadet P, Mantione KJ, Stefano GB (2005) Morphine via nitric oxide modulates beta-amyloid metabolism: a novel protective mechanism for Alzheimer’s disease. Med Sci Monit 11:BR357–BR366
Ripoli C, Piacentini R, Riccardi E, Leone L, Li Puma DD, Bitan G, Grassi C (2013) Effects of different amyloid β-protein analogues on synaptic function. Neurobiol Aging 34:1032–1044
Stewart VC, Heales SJ (2003) Nitric oxide-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic Biol Med 34:287–303
Tang BL (2005) Alzheimer’s disease: channeling APP to non-amyloidogenic processing. Biochem Biophys Res Commun 331:375–378
Tienari PJ, Ida N, Ikonen E, Simons M, Weidemann A, Multhaup G, Masters CL, Dotti CG, Beyreuther K (1997) Intracellular and secreted Alzheimer beta-amyloid species are generated by distinct mechanisms in cultured hippocampal neurons. Proc Natl Acad Sci USA 94:4125–4130
Wang JZ, Zhang Y (2015) Configuration-specific immunotherapy targeting cis pThr231-Pro232 tau for Alzheimer disease. J Neurol Sci 348:253–255
Wang JZ, Li SR, Li YL, Zhang YZ, Zhang T, Zhao CX, Yao CX, Du LF (2013) Could Pin1 help us conquer essential hypertension at an earlier stage? A promising early-diagnostic biomarker and its therapeutic implications for the disease. Med Hypotheses 81:931–935
Wang JZ, Zhu WD, Xu ZX, Du WT, Zhang HY, Sun XW, Wang XH (2014) Pin1, endothelial nitric oxide synthase, and amyloid-β form a feedback signaling loop involved in the pathogenesis of Alzheimer’s disease, hypertension, and cerebral amyloid angiopathy. Med Hypotheses 82:145–150
Westermann B (2009) Nitric oxide links mitochondrial fission to Alzheimer’s disease. Sci Signal 2:pe29
Acknowledgments
This work was supported by the National Basic Research Program of China (2013CB531703), the National Natural Science Foundation of China (NSFC 81370470, 81202448, 11204023, and 81273919), the Humanity and Social Science Research Funds of Ministry of Education (13YJCZH087), the Scientific Research Fund of Liaoning Provincial Education Department (L2015145), and the Science and Technology Project of Liaoning Province (2012225020).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None of the authors have actual or potential conflicts of interest. None of the authors’ institution has contracts relating to this research. The authors indicated that they and their institutions have no other agreements that could be seen as involving a financial interest in this work.
Additional information
Zheng-Xu Cai, Hui-Shu Guo, and Che Wang contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12640_2015_9564_MOESM1_ESM.pptx
Supplement Fig. 1. Various concentrations of SNP on cells proliferation as determined by MTT assay. SNP at higher concentrations (50 to 200 μM) induced a significant cytotoxicity, whereas SNP at lower concentrations (0.01 and 0.1 μM) have the tendency to promote cells proliferation (a); the IC50 value was calculated on the basis of MTT assay using GraphPad Prism (b). Supplementary material 1 (PPTX 56 kb)
Rights and permissions
About this article
Cite this article
Cai, ZX., Guo, HS., Wang, C. et al. Double-Edged Roles of Nitric Oxide Signaling on APP Processing and Amyloid-β Production In Vitro: Preliminary Evidence from Sodium Nitroprusside. Neurotox Res 29, 21–34 (2016). https://doi.org/10.1007/s12640-015-9564-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-015-9564-6