Abstract
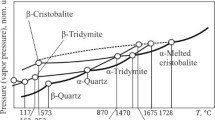
A gray-colored sediment from Erzurum/Turkey zone was characterized by scanning electron microscopy, X-ray diffraction, chemical analysis, and thermal analysis techniques. The raw sample contains mainly an amorphous biogenic opal-A as well as opal-CT, smectite, illite, and quartz as impurities. The XRD-pattern of the heated samples at different temperatures ranging between 1050 and 1175oC for durations from zero to 4 h was recorded. Consecutive increases in the intensity of the characteristic XRD-reflection at 2θ = 22o showed that the biogenic silica changed over paracrystalline opal-CT to crystalline α-cristobalite having the reflection with the maximum intensity (Im). Its insolubility in orthophosphoric acid digestion proved this formation. The temperature and heating time dependent intensity (I) were used as kinetic variables and the ratio of \( I/{I}_m=\alpha \)was defined as crystalline fraction. Using the differential rate law, \(d\alpha /dt=k {(1-\alpha )}^{n}\) and Arrhenius equation \(\text{ln}k=-{E}^{\#}/RT+ln A\), the order and activation energy for the crystallization were calculated as \(n=1\), and \({E}^{\#}=202 \text{k}\text{J}{ \text{m}\text{o}\text{l}}^{-1},\) respectively.
Similar content being viewed by others
Data Availability
The authors declare that the authenticity of their own collected data and materials used in this paper is guaranteed.
References
Dress LR, Wilding LP, Smeck NE, Senkayi AL (1995) Silica in soils: Quartz and disordered silica polymorphs. Minerals in Soil Environments, 2nd edn. Soil Science Society of America, Madison
Iller RK (1978) The Chemistry of Silica. Wiley, New York
Miles WJ (1994) Crystalline silica analysis of Wyoming bentonite by X-ray diffraction after phosphoric acid digestion. Anal Chim Acta 286:97–105
Kahraman S, Önal M, Sarıkaya Y, Bozdoğan İ (2005) Characterization of silica polymorphous in kaolins by X-ray diffraction before and after phosphoric acid digestion and thermal treatment. Anal Chim Acta 552:201–206
Elzea JM, Rice SB (1996) TEM and X-ray diffraction evidence for cristobalite and tridymite stocking sequences in opal. Clays Clay Miner 44:492–500
Yılmaz H, Kaçmaz H (2012) Distinguishing opaline silica polymorphs from α-cristobalite in Gedikler bentonite (Uşak, Turkey). Appl Clay Sci 62–63:80–86
Chao CH, Lu HY (2002) Stress-induced β→α-cristobalite phase transformation in (Na2O+Al2O3)-codoped silica. Mater Sci Eng 328:267–276
Jones JB, Segnit ER (1971) The nature of opal. I. Nomenclature and constituent phases. J Geol Soc Aust 8:57–68
Elzea JM, Odom IE, Miles WI (1994) Distinguishing well ordered opal-CT and opal-C from high temperature cristobalite by X-ray diffraction. Anal Chim Acta 286:107–116
Nagase T, Akizuki M (1997) Texture and structure of opal-CT and opal-C in volcanic rocks. Can Mineral 35:947–958
Jones JB, Sanders JV, Segnit ER (1964) Structure of opal. Nature 64:990–991
Yuan Jia, Wang B (2017) Mineralogy and thermal analysis of natural pozzalona opal shale with nanopores. J Wuhan Univ Technol Mater Sci Ed 32:532–537
Fröhlich F (2020) The opal-CT nanostructure. J Non-Crystalline Solids 533:119938
Bustillo MA, Martinez-Frias J (2003) Green opals in hydrothermalized basalts (Tenerife Island, Spain): alteration and aging of silica pseudoglass. J Non-Cryst Solids 323:27–33
Ostrooumov M (2007) A Raman, Infrared and XRD analysis of the instability in volcanic opals from Mexico. Spectrochim Acta Part A 68:1070–1076
Chauvire B, Thomas PS (2020) DSC of natural opal: insight into the incorporation of crystallisable water in the opal microstructure. J Therm Anal Calorim 140:2077–2085
Banerjee A, Wenzel T (1999) Black opal from Honduras. Eur J Mineral 11:401–408
Brown LD, Ray AS, Thomas PS (2003) 29Si and 27Al NMR study of amorphous and paracrystalline opals from Australia. J Non-Cryst Solids 332:242–248
Clarke J (2003) The occurrence and significance of biogenic opal in the regolith. Earth Sci Rev 60:175–194
Meunier ID, Colin F, Alarcon C (2015) Biogenic silica storage in soils. Geology 27:835–838
Golubev VG, Hutchson JL, Kosobukin VA, Kurdyukov DA, Medvedev AV, Pevtsov AB, Slon J, Sorokin LM (2002) Three-dimensional ordered silicon-based nanostructures in opal matrix: preparation and photonic properties. J Non-Cryst Solids 299–302:1062–1069
Dubrovinsky LS, Dubrovinskaia NA, Prakopenka V, Seifert F, Langenhorst F, Dmitriev V, Weber HP, Le Bihan T (2004) A class of new high-pressure silica polymorphs. Phys Earth Planet Inter 143–144:231–240
Shöf O, Ghobarkar H, Ganier A, Vagner C, Linder JKN, Hans J, Reller A (2006) Synthesis of nanocrystalline low temperature silica polymorphs. Solid State Sci 8:625–633
Ruhl T, Spahn P, Hellmann GP (2003) Artificial opals prepared by melt compression. Polymer 44:7625–7634
Mitchell LD, Beaudoin JJ, Grattan-Bellew PE (2004) The effect lithium hydroxide solution on alkali silica reaction gels created with opal. Cem Concr Res 34:641–649
Bordosova M, Hodge P, Pach l, Pemble ME, Smata V, Tredgold RH, Whitehed D (2003) Synthetic opals made by the Langmuir-Blodgett method. Thin Solid Films 437:276–279
Temuujin J, Okado K, MacKenzie KJD (2003) Preparation of porous silica from vermiculite by selective leaching. Appl Clay Sci 22:187–195
Kato N, Kato N (2016) High-yield hydrothermal synthesis of mesoporous silica hollow capsules. Micropor Mesopor Mater 219:230–239
Beygi H, Kerimi EZ, Farazi R, Ebrahimi F (2016) A statistical approach to synthesis of functionally modified silica nanoparticles. J Alloys Compd 654:308–314
Krasucha P, Stefaniak W, Kierys A, Goworek J (2016) One-pot synthesis of two different highly Porous silica materials. Micropor Mesopor Mater 221:14–25
Rouquerol F, Rouquerol J, Sing KSW, Llewellyn P, Maurin G (2014) Adsorption by Powders and porous Solids, 2nd edn. Elsevier, Amsterdam
Romanov SG, Ferrand P, Eger M, Zentel R, Ahopelto J, Gaponik N, Eychmüller A, Rogach AL, Jorres CMS (2003) Exploring integration prospects of opal-based photonic crystals. Synth Met 139:701–704
Garcia G, Cardenas E, Cabrera S, Hedlund J, Mouzon J (2016) Synthesis of zeolite Y from diatomite as silica source. Micropor Mesopor Mater 219:29–37
Sah RP, Choudhury B, Das RK (2015) A review on adsorption coaling systems with silica gel and carbon as adsorbents. Renew Sust Energy Rev 45:123–134
Wong JCH, Kaymak H, Brunner S, Koebel MM (2015) Mechanical and thermal properties of nanofibrillated cellulose reinforced silica aerogel composites. Micropor Mesopor Mat 217:150–158
Mignot M, Sebban M, Tchopla A, Mercier O, Cardinael P, Peulon Agasse V (2015) Thermal pretreatments of superficially porous silica particles for high-performance liquid chromatography: surface control, structural characterization and chromatographic evaluation. J Chromatogr A 1419:45–57
Hu X, Yan X, Zhou M, Komarneni S (2016) One-step synthesis of nanostructural mesoporous ZIF-8/silica composites. Micropor Mesopor Mater 219:311–316
Scherer GW (1997) Effect of drying on properties of silica gel. J Non-Cryst Solids 215:155–168
Arasuma A, Okuma M, Okudera H, Mizukami T, Arai S, Katayama S, Koyamo M, Ito N (2013) Structural changes of synthetic opal by heat treatment. Phys Chem Miner 40:747–755
Ceylan H, Pekdemir AD, Önal M, Sarıkaya Y (2021) The effect of the hydrothermal and thermal deactivations on the adsorptive properties and liquid permeability of a silica gel. JOTCSA 8:477–482
Önal M, Kahraman S, Sarıkaya Y (2007) Differential of α-cristobalite from opals in bentonites from Turkey. Appl Clay Sci 35:25–30
Önal M, Sarıkaya Y (2007) The effect of heat treatment on the paracrystallinity of an opal-CT found in a bentonite. J Non-Cryst Solids 353:4195–4198
Gislason SR, Heaney PJ, Celkers EH, Scholt J (1997) Kinetic and thermodynamic properties of moganite, a novel silica polymorphs. Geochim Cosmochim 61:1193–1204
Vyazovkin S, Burnham AK, Criado JM, Perez-Maquedo LA (2011) ICTAC Kinetics Committee recommendation for performing kinetic computations on thermal analyses data. Thermochim Acta 520:1–19
Noyan H, Önal M, Sarıkaya Y (2008) A model developed for acid dissolution thermodynamics of a Turkish bentonite. J Therm Anal Calorim 94:591–596
Pekdemir AD, Sarıkaya Y, Önal M (2015) Thermal transformation kinetics of a kaolinitic clay. J Therm Anal Calorim 123:767–777
Sarıkaya Y, Önal M (2016) An indirect model for sintering thermodynamics. Turkish J Chem 40:841–845
Sarıkaya Y, Ceylan H, Önal M, Pekdemir AD (2021) Thermal deactivation kinetics and thermodynamics of a silica gel using surface area data. J Therm Anal Calorim 146:1505–1510. https://doi.org/10.1007/s10973-020-10132-z
Sarıkaya Y, Önal M, Pekdemir AD (2019) Application of diffusion and transition state theories on the carburizing of a steel. AISI 316 by annealing in uranium carbide powder. Heliyon 5:e02305
Sarıkaya Y, Önal M, Pekdemir AD (2020) Thermal degradation kinetics of sepiolite. Clay Miner 55:96–100
Sarıkaya Y, Önal M, Pekdemir AD (2020) Kinetic and thermodynamic approaches on thermal degradation of sepiolite crystal using XRD-analysis. J Therm Anal Calorim 140:2667–2672
Warr LN (2020) Recommended abbreviations for the names of clay minerals and associated phases. Clay Miner 55:261–264
Acknowledgements
Authors would like acknowledge the funding from Ankara University Scientific Research Projects Coordination Unit (19L0430007) for conducting this research.
Funding
This work received financial support from Ankara University Scientific Research Projects Coordination Unit (19L0430007).
Author information
Authors and Affiliations
Contributions
The signed authors have all participated in the different parts of this paper. S. Daglar: Running Experiments, Investigation. N. D. Kahya: Characterization and formal analysis. G. Ustunisik: Visualization, Provide ideas, Writing, Revisions and Editing. M. Onal: Supervision, Analysis, Visualization, Writing, Conceptualization, Funding acquisition. Y. Sarikaya: Co-supervision, Methodology, Writing - original draft.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The authors declare that they comply with ethical standards.
Consent for Publication
The authors declare that the consent to the publication of the relevant data is granted.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dağlar, S., Kahya, N.D., Ustunisik, G. et al. Thermal Crystallization Kinetics of an Opal-like Biogenic Silica. Silicon 14, 7211–7217 (2022). https://doi.org/10.1007/s12633-021-01498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01498-2