Abstract
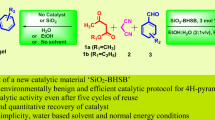
Silica supported acids like Si-KHSO4, and Si-HClO4 are explored as reusable nano green catalysts for bromination of aromatic and hetero aromatic compounds using KBr under solvothermal, and solvent-free conditions. Reaction times reduced from (4–6) hours under conventional solvothermal protocols to (9–12) minutes under ultrasonic sonucation for completion. But, solvent-free microwave assisted reactions required only (1–5) minutes exhibiting striking rate accelerations compared to the solvothermal and ultrasonic assisted protocols. All the reaction protocols afforded fairly good yields of brominated products, which are comparable with existing protocols.
Similar content being viewed by others
References
Brown RS (1997) Investigation of the early steps in electrophilic Bromination through the study of the reaction with sterically encumbered olefins. Acc Chem Res 30(3):131–137. https://doi.org/10.1021/ar960088e
Larock RC (1999) Comprehensive organic transformations2nd edn. Wiley-VCH, New York
Podgor-sek A, Zupan M, Iskra J (2009) Oxidative halogenation with “green” oxidants: Oxygen and hydrogen peroxide. Angew Chem Int 48(45):8424–8450. https://doi.org/10.1002/anie.200901223
Bora U, Chaudhuri MK, Dey D, Dhar SS (2001) Peroxometal-mediated environmentally favorable route to brominating agents and protocols for bromination of organics. Pure Appl Chem 73(1):93–102. https://doi.org/10.1351/pac200173010093
Srivastava SK, Chauhan PMS, Bhaduri AP (1996) Novel site-specific one-step bromination of substituted benzenes. Chem Commun 23:2679–2680. https://doi.org/10.1039/CC9960002679
Clark JH, Ross JC, Macquarrie DJ, Barlow SJ, Bastock TW (1997) Environmentally friendly catalysis using supported reagents: the fast and selective bromination of aromatic substrates using supported zinc bromide1. Chem Commun 13:1203–1204. https://doi.org/10.1039/A702399E
Smith K, El-Hiti GA, Hammond MEW, Bahzad D, Li Z, Siquet C (2000) Highly efficient and selective electrophilic and free radical catalytic bromination reactions of simple aromatic compounds in the presence of reusable zeolites. J Chem Soc Perkin Trans 1(16):2745–2752. https://doi.org/10.1039/B002157L
Schmid H (1946) Bromierungen mit Brom-succinimid bei Gegenwart von Katalysatoren, II. Helv Chim Acta 29(5):1144–1151. https://doi.org/10.1002/hlca.19460290520
Duan J, Zhang LH, Dolbier Jr WR (1999) A convenient new method for the Bromination of deactivated aromatic compounds. Synlett. 8:1245–1246. https://doi.org/10.1055/S-1999-2818
Bovonsombat P, McNelis E (1993) Ring halogenations of Polyalkylbenzenes with N-Halosuccinimide and acidic catalysts. Synthesis 2:237–241. https://doi.org/10.1055/s-1993-25839
Auerbach J, Weissman SA, Blacklock TJ, Angeles MR, Hoogsteen.K. (1993) N-Bromosuccinimide/Dibromodimethylhydantoin in aqueous base: A practical method for the bromination of activated benzoic acids. Tetrahedron Lett 34(6):931–934. https://doi.org/10.1016/S0040-4039(00)77457-0
Konishi VH, Aritomi K, Okano T, Kiji J (1989) A Mild Selective Monobromination Reagent System for Alkoxybenzenes; N-Bromosuccinimide–Silica Gel. Bull Chem Soc Jpn 62(2):591–593. https://doi.org/10.1246/bcsj.62.591
Ranu BC, Sarkar DC, Chakraborty R (1992) A simple and improved procedure for selective ring Bromination of alkyl-substituted aromatic hydrocarbons on the surface of alumina. Synth Commun 22(8):1095–1099. https://doi.org/10.1080/00397919208021092
Vega F, Sasson Y, Huddersman K (1993) Highly selective bromination of toluene in a bromine—oxirane—zeolite system. Zeolites 13(5):341–347. https://doi.org/10.1016/0144-2449(93)90148-V
Smith K, Bahzad D (1996) Highly efficient Para-selective bromination of simple aromatic substrates by means of bromine and a reusable zeolite. J Chem Commun 4:467–468. https://doi.org/10.1039/CC9960000467
Goldberg Y, Alper H (1994) Electrophilic halogenation of aromatics and heteroaromatics with N-halosuccinimides in a solid/liquid system using an H+ ion exchanger or ultrasonic irradiation. J Mol Catal 88(3):377–383. https://doi.org/10.1016/0304-5102(93)E0278-O
Paul V, Sudalai A, Daniel T, Srinivasan KV (1994) Regioselective bromination of activated aromatic substrates with N-bromosuccinimide over HZSM-5. Tetrahedron Lett 35(38):7055–7056. https://doi.org/10.1016/0040-4039(94)88224-X
Barhate NB, Gajare AS, Wakharkar RD, Bedekar AV (1998) Simple and efficient chlorination and bromination of aromatic compounds with aqueous TBHP (or H2O2) and a hydrohalic acid. Tetrahedron Lett 39(35):6349–6350. https://doi.org/10.1016/S0040-4039(98)01305-7
Majetich G, Hicks R, Reister S (1997) Electrophilic aromatic Bromination using Bromodimethylsulfonium bromide generated in situ. J Organomet Chem 62(13):4321–4326. https://doi.org/10.1021/jo970135w
Voskressensky LG, Golantsov NE, Maharramov AM (2016) Recent advances in Bromination of aromatic and Heteroaromatic compounds. Synthesis 48(05):615–643. https://doi.org/10.1055/s-0035-1561503
Levy JN, Alegre-Requena JV, Liu R, Paton RS, McNally A (2020) Selective halogenation of pyridines using designed phosphine reagents. J Am Chem Soc 142(25):11295–11305. https://doi.org/10.1021/jacs.0c04674
Das B, Venkateswarlu K, Krishnaiah M, HarishHolla (2006) An efficient, rapid and regioselective nuclear bromination of aromatics and heteroaromatics with NBS using sulfonic-acid-functionalized silica as a heterogeneous recyclable catalyst. Tetrahedron Lett 47(49):8693–8697. https://doi.org/10.1016/j.tetlet.2006.10.029
Singhal S, Jain SL, Sain B (2006) A simple and improved regioselective bromination of aromatic compounds using N-methylpyrolidin-2-one hydrotribromide and aqueous hydrogen peroxide under mild reaction conditions. J Mol Catal A Chem 258(1–2):198–202. https://doi.org/10.1016/j.molcata.2006.05.042
Artasensi A, Pedretti A, Vistoli G, Fumagalli L (2021) Regioselective, efficient and sustainable Bromination process for the synthesis of the antimicrobial agent Bromiphen bromide. Org Prep Proced Int 53(5). https://doi.org/10.1080/00304948.2021.1956849
Chaudhuri SK, Sanchita R, Saha M, Bhar S (2007) Regioselective aromatic electrophilic Bromination with Dioxane dibromide under solvent-free conditions. Synth Commun 37(4):579–583. https://doi.org/10.1080/00397910601055081
Wortel TM, Oudijn D, Vleugel CJ, Roelofsen DP, van Bekkum H (1979) Selective bromination of halobenzenes using zeolite catalysts. J Catal 60(1):110–120. https://doi.org/10.1016/0021-9517(79)90073-3
Asgari Bajgirani M (2021) Application of Ziegler-Nata catalysts in the synthesis of polyolefin. Prog Chem Biochem Res 4(1):20–31. https://doi.org/10.22034/pcbr.2021.118046
Moosavi-Zare AR, Goudarziafshar H, Jalilian Z (2019) Tandem Knoevenagel-Michael-cyclocondensation reaction of malononitrile, various aldehydes and barbituric acid derivatives using isonicotinic acid as an efficient catalyst. Prog Chem Biochem Res 2(2):59–65. https://doi.org/10.33945/SAMI/PCBR.2019.2.2.3
Jalilian R, Shahmari M, Taheri A, Gholami K (2020) Ultrasonic-assisted micro solid phase extraction of arsenic on a new ion-imprinted polymer synthesized from chitosan-stabilized Pickering emulsion in water, rice and vegetable samples. Ultrason Sonochem 61:104802. https://doi.org/10.1016/j.ultsonch.2019.104802
Mukut G, van Tonder JH, Benzuidenhoudt BCB (2015) NaHSO4-SiO2: An efficient reusable green catalyst for selective C-3 Propargylation of indoles with tertiary propargylic alcohols. Iran J Chem Chem Eng 34(3):11–17. https://doi.org/10.30492/ijcce.2015.14747
Azarifar D, Forghaniha A (2006) A novel Chemoselective reaction of aldehydes with 2-Mercaptoethanol catalyzed by SiO2-NaHSO4 under solvent-free condition. J Chin Chem Soc 53(5):1189–1192. https://doi.org/10.1002/jccs.200600157
Mishra S, Ghosh R (2011) Mechanistic studies on a new catalyst system (CuI-NaHSO4×SiO2) leading to the one-pot synthesis of Imidazo[1,2-a] pyridines from reactions of 2-Aminopyridines, aldehydes, and terminal alkynes. Synthesis. 21:3463–3470. https://doi.org/10.1055/s-0030-1260255
Kinfe HH, Mebrahtu FM, Moshapo PT (2013) Solvent-free NaHSO4-SiO2-catalyzed efficient Tetrahydropyranylation of alcohols and phenols. Synth Commun 43(9):1237–1242. https://doi.org/10.1080/00397911.2011.629068
Siddiqui ZN, Farooq F (2012) Silica supported sodium hydrogen sulfate (NaHSO4–SiO2): A novel, green catalyst for synthesis of pyrazole and pyranyl pyridine derivatives under solvent-free condition via heterocyclic β-enaminones. J Mol Catal A Chem 363-364:451–459. https://doi.org/10.1016/j.molcata.2012.07.024
Maghsoodlou MT, Heydari R, Habibi-Khorassani SM, Hazeri N, Sajadikhah SS, Rostamizadeh M, Lashkari M (2012) One-pot, three-component synthesis of α-amino phosphonates using NaHSO4-SiO2 as an efficient and reusable catalyst. Synth Commun 42(1):136–143. https://doi.org/10.1080/00397911.2010.523153
Chari MA, Syamasundar K (2004) Silicagel supported sodium hydrogensulfate as a heterogenous catalyst for high yield synthesis of 3,4-dihydropyrimidin-2 (1H)-ones. J Mol Catal A Chem 221(1–2):137–139. https://doi.org/10.1016/j.molcata.2004.06.019
Adharvana Chari M, Syamasundar (2005) Silica gel/NaHSo4 catalyzed one-pot synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Catal Commun 6(9):624–626. https://doi.org/10.1016/j.catcom.2005.03.010
Das B, Ravikanth B, Laxminarayana K, VittalRao B (2006) A simple and facile synthesis of homoallylic amines using silica supported sodium hydrogen sulfate. J Mo Catal A: Chemical 253(1–2):92–95. https://doi.org/10.1016/j.molcata.2006.03.007
Das B, Banerjee J (2004) Silica-supported Sodium Hydrogen Sulfate and Amberlyst-15: Two Efficient Heterogeneous Catalysts for Single-step Synthesis of 4(3H)-Quinazolinones from Anthranilic Acid, Ortho Esters, and Amines under Solvent Free Conditions. Chem Lett 33(8):960–961. https://doi.org/10.1246/cl.2004.960
Khan AT, Choudhury LH, Ghosh S (2006) Silica supported perchloric acid (HClO4-SiO2): A highly efficient and reusable catalyst for geminal diacylation of aldehydes under solvent-free conditions. J Mo Catal A: Chemical 255(1–2):230–235. https://doi.org/10.1016/j.molcata.2006.04.008
Bigdeli MA, Heravi MM, Mahdavinia GH (2007) Silica supported perchloric acid (HClO4-SiO2): A mild, reusable and highly efficient heterogeneous catalyst for the synthesis of 14-aryl or alkyl-14-H-dibenzo[a,j]xanthenes. J Mo Catal A: Chemical 275(1–2):25–29. https://doi.org/10.1016/j.molcata.2007.05.007
Bigdeli MA, Nemati F, Mahdavinia GH (2007) HClO4–SiO2 catalyzed stereoselective synthesis of β-amino ketones via a direct Mannich-type reaction. Tetrahedron Lett 48(38):6801–6804. https://doi.org/10.1016/j.tetlet.2007.07.088
Bandgar BP, Gawande SG, Muley DB (2010) Silica supported perchloric acid (HClO4-SiO2): A green, reusable, and highly efficient heterogeneous catalyst for the synthesis of thioethers under solvent-free conditions at room temperature. Green Chem Lett Rev 3(1):49–54. https://doi.org/10.1080/17518250903447118
Maheswara M, Siddaiah V, Damu GLV, Rao CV (2006) An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using heterogeneous catalyst under solvent-free conditions. ARKIVOC 2006(ii):201–206. https://doi.org/10.3998/ark.5550190.0007.223
Yuguo D, Wei G, Cheng S, Hua Y, Linhardt RJ (2006) HClO4–SiO2 catalyzed glycosylation using sugar trichloroacetimidates as glycosyl donors. Tetrahedron Lett 47:307–310. https://doi.org/10.1016/j.tetlet.2005.11.025
Murthy YLN, Diwakar BS, Govindh B, Venu R, Nagalakshmi K (2013) Silica Perchloric acid matrix supported ring opening of epoxide under microwave radiation. ChemSci Trans 2(3):805–812. https://doi.org/10.7598/cst2013.471
Choudary BM, Sudha Y, Reddy PN (1994) Regioselective Oxybromination of activated aromatic compounds catalysed by ammonium Molybdate. Synlett. 6:450. https://doi.org/10.1055/s-1994-22886
Bandgar BP, Nigal NJ (1998) Regioselective Catalytic Halogenation of Aromatic Substrates. Synthetic Com 28(17):3225–3229. https://doi.org/10.1080/00397919808004426
Tamhankar BV, Desai UV, Mane RB, Wadgaonkar PP, Bedekar AV (2001) A simple and practical halogenation of activated arenes using potassium halide and oxone in water-acetonitrile medium. Synthetic Com 31(13):2021–2027. https://doi.org/10.1081/SCC-100104419
Narender N, Srinivasu P, Ramakrishna Prasad M, Kulkarni SJ, Raghavan KV (2002) An efficient and regioselective oxybromination of aromatic compounds using potassium bromide and oxone. Synthetic Com 32(15):2313–2318. https://doi.org/10.1081/SCC-120006001
Reza HA, MallakpourShadpour E, AdibiHadi (2000) Benzyltriphenylphosphonium Peroxymonosulfate: as a novel and efficient reagent for oxidation of alcohols under solvent-free conditions. Chem Lett 29(5):460–461. https://doi.org/10.1246/cl.2000.460
Hajipour AR, Mallakpour SE, Adibi H (2001) Oxidation of Urazoles to Triazolinediones with Benzyltriphenylphosphonium Peroxymonosulfate under solvent-free conditions. Chem Lett 30(2):164–165. https://doi.org/10.1246/cl.2001.164
Hajipour AR, Mallakpour SE, Baltork IM, Adibi H (2001) Conversion of oximes, phenylhydrazones, 2,4-dinitrophenylhydrazones, and semicarbazones to corresponding carbonyl compounds with benzyltriphenylphosphonium peroxymonosulfate (bnph3p+hso5 −) (btppms) in the presence of bismuth chloride under non-aqueous conditions. Synth Commun 31(22):3401–3409. https://doi.org/10.1081/SCC-100106197
Hajipour AR, Mallakpour SE, Adibi H (2002) A selective solid-state oxidation of sulfides and thiols with Benzyltriphenylphosphonium Peroxymonosulfate. Phosphorus Sulfur Silicon 177(10):2277–2284. https://doi.org/10.1080/10426500214100
Hajipour AR, Mallakpour SE, Baltork IM, Adibi H (2002) A convenient method for Dethioacetalization of 1,3-Dithiolanes and 1,3-Dithianes using Benzyltriphenylphosphonium Peroxymonosulfate in aprotic solvent. Phosphorus Sulfur Silicon 177(12):2805–2811. https://doi.org/10.1080/10426500214884
Siddiqui ZN (2019) A convenient synthesis of coumarinyl chalcones using HClO4–SiO2: a green approach. Arab J Chem 12(8):2788–2797. https://doi.org/10.1016/j.arabjc.2015.06.013
Hemanth Sriram Y, Fatima T, Satish Kumar M, Rajanna KC, Venkateswarlu M, Sai Sudhakar M, Madhusudan Raju R (2017) Reusable silica supported perchloric acid and potassium bisulphate as green catalysts for thiocyanation of aromatic compounds under solvent free conditions. Iran Chem Commun 5(3):352–363
Fatima T, Hemanth Sriram Y, Satish Kumar M, Venkateswarlu M, Rajanna KC (2017) Silica-supported HClO4 and KHSO4 as reusable green catalysts for sulfonation of aromatic compounds under solvent-free conditions. Asian J Green Chem 1(2):69–77. https://doi.org/10.22631/ajgc.2017.95574.1016
Anastas P, Warner J (1998) In Green Chemistry: Theory and Practice. Oxford University Press, New York ISBN:0198502346, 9780198502340
Mason TJ, Lorimer JP (1989) Sonochemistry: theory, applications and uses of ultrasound in chemistry. Ellis Horwood Ltd 93(10):1150–1151. https://doi.org/10.1002/bbpc.19890931025
Suslick, K.S. (1988) Ultrasound, it’s chemical, physical and biological effects. VCH Publishers, Inc.
Singh V, Kaur KP, Khurana A, Kad GL (1998) Ultrasound: A boon in the synthesis of organic compounds. Resonance 3(9):56–60. https://doi.org/10.1007/BF02836081
Polshettiwar V, Varma RS (2008) Aqueous microwave chemistry: A clean and green synthetic tool for rapid drug discovery. Chem Soc Rev 37(8):1546–1557. https://doi.org/10.1039/B716534J
Lidström P, Tierney J, Wathey B, Westman J (2001) Microwave assisted organic synthesis—a review. Tetrahedron. 57(45):9225–9283. https://doi.org/10.1016/S0040-4020(01)00906-1
Varma RS (1999) Solvent-free organic syntheses . Using supported reagents and microwave irradiation. Green Chem 1(1):43–55. https://doi.org/10.1039/A808223E
Oliver Kappe C (2004) Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 43(46):6250–6284. https://doi.org/10.1002/anie.200400655
Nath J, Chaudhuri MK (2008) Boric acid catalyzed bromination of a variety of organic substrates: an eco-friendly and practical protocol. Green Chem Lett Rev 1:223–230
Venkateswarlu, K., Suneel, K., Das,B., Nagabhushana Reddy, K.,Sreenivasulu Reddy, T.: Simple -free Regio- and Chemoselective Monobromination of aromatics using NBS in polyethylene glycol, Synth Commun, 2008, 39, 215–219
Dey RR, Dhar SS (2013) Ammonium persulphate promoted synthesis of polyethylene glycol entrapped potassium tribromide and its application in acylation and bromination of some selective organic compounds. ChinChem Lett 24:866–868
Ma X, Yu J, Jiang M, Wang M, Tang L, Wei M, Zhou Q (2019) Mild and Regioselective Bromination of phenols with TMSBr. Eur J Org Chem. https://doi.org/10.1002/ejoc.201900794
Chakradhar A, Roopa R, Rajanna KC, Saiprakash PK (2009) Vilsmeier-Haack Bromination of aromatic compounds with KBr and N-Bromosuccinimide under solvent-free conditions. Synth Commun 39:1817–1824
Acknowledgements
Authors gratefully acknowledge Head, Department of Chemistry, Osmania University, Hyderabad for facilities. Authors are grateful to Professor P. K. Saiprakash (Former Dean, Faculty of Science, O.U)), and Prof. Authors are grateful to Professor P. K. Saiprakash (Former Dean, Faculty of Science, O.U)), and Prof. T.Navaneeth Rao (former Vice-chancellor, O.U), for constant encouragement. Authors are thankful to CSIR-IICT, Hyderabad, and Central Instrumentation Centre, O.U. for providing spectroscopic and BET studies.
Availability of the Data and Materials
All the data emodied in this manuscript are prepared by following the research ethics, and it may be avialable to th readers after publication according the conditions of Publishers of this journal.
Funding
Funding is not reecieved specifically for this work. However, one of the authors (Vijay Shekar Pulusu) is highly thankful to CSIR, New Delhi for the award of Junior Research Fellowship.
Author information
Authors and Affiliations
Contributions
Not Applicable.
Corresponding author
Ethics declarations
Authors declare that none of them have any conflict of interest.
Authors Consent to Participate
All the authors participated enthusiastically in the experimental work and also the prepartion of manuscript under the guidance of Corresponding author (Prof. K. C. Rajanna). We have followed all ethics neeeded for reeseach activity in our laboratory. This part of the research work does not involve either Human Participants and/or Animals as tools in case studies.
Authors Consent for Publication
The authors herey declare that the work is original and solely submitted only to this journal. It is not submitted any where. All the funding bodies, and institutions are gratefully acknowledged appropriately.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pulusu, V.S., Kamatala, C.R., Mardhanpally, A.K. et al. Silica Supported Acids (SiO2-HClO4, SiO2-KHSO4) as Eco-Friendly Reuasble Catalysts for Bromination of Aromatic and Heteroaromatic Compounds Using KBr under Solvothermal and Solvent-Free Conditions. Silicon 14, 7781–7791 (2022). https://doi.org/10.1007/s12633-021-01489-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01489-3