Abstract
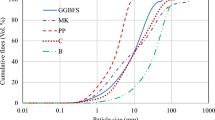
Kaolin samples obtained from Cameroon were used to produce geopolymer binders. Prior to its application, the raw kaolin samples were activated through the gliding arc plasma treatment using both spatial post-discharge and direct mode. A mixture of sodium hydroxide and silicate was used as the alkaline solution. In order to study the influence of the modifications generated by the gliding arc plasma treatment on the geopolymerization process, X-ray diffraction, thermogravimetric analysis, differential scanning calorimeter and Fourier transform infrared spectroscopy were carried out. In addition, scanning electron microscopy, nitrogen physisorption and compression tests analysis were also carried out on the resulting geopolymer samples to access their mechanical performance. The results showed that the geopolymerization process was not completed at the curing temperature of 90 °C. Plasma spatial post-discharge mode treated kaolin led to 20.48% increase in compressive strength when compared with the geopolymer prepared from raw kaolin.
Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this article.
References
Davidovits J (1991) Geopolymers - inorganic polymeric new materials. J Therm Anal 37:1633–1656. https://doi.org/10.1007/BF01912193
Abbas R, Al Khereby M, Ghorab HY, Elkhoshkhany N (2020) Preparation of geopolymer concrete using Egyptian kaolin clay and the study of its environmental effects and economic cost. Clean Techn Environ Policy 22:669–687. https://doi.org/10.1007/s10098-020-01811-4
Komnitsas K, Zaharaki D (2007) Geopolymerisation : a review and prospects for the minerals industry. Miner Eng 20:1261–1277. https://doi.org/10.1016/j.mineng.2007.07.011
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, Van Deventer JSJ (2007) Geopolymer technology : the current state of the art. J Mater Sci 42:2917–2933. https://doi.org/10.1007/s10853-006-0637-z
Davidovits J (2011) Geopolymer chemistry and applications3rd edn. Institut Géopolymère, France
Van Deventer JSJ, Provis JL, Duxson P, Lukey GC (2007) Reaction mechanisms in the geopolymeric conversion of inorganic waste to useful products. J Hazard Mater 139:506–513. https://doi.org/10.1016/j.jhazmat.2006.02.044
Naghizadeh A, Ekolu SO (2020) Effects of compositional and Physico – chemical mix design parameters on properties of Fly ash Geopolymer mortars. Silicon. https://doi.org/10.1007/s12633-020-00799-2
Meftah N, Mahboub MS (2020) Spectroscopic characterizations of sand dunes minerals of El-Oued (northeast Algerian Sahara) by FTIR, XRF and XRD analyses. Silicon. 12:147–153. https://doi.org/10.1007/s12633-019-00109-5
Tchadjie LN, Ekolu SO (2018) Enhancing the reactivity of aluminosilicate materials toward geopolymer synthesis. J Mater Sci 53:4709–4733. https://doi.org/10.1007/s10853-017-1907-7
Xu H, Van Deventer JSJ (2000) The geopolymerisation of alumino-silicate minerals. Int J Miner Process 59:247–266. https://doi.org/10.1016/S0301-7516(99)00074-5
Barbosa VFF, MacKenzie KJD, Thaumaturgo C (2000) Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: sodium polysialate polymers. Int J Inorg Mater 2:309–317. https://doi.org/10.1016/S1466-6049(00)00041-6
Elimbi A, Tchakoute HK, Njopwouo D (2011) Effects of calcination temperature of kaolinite clays on the properties of geopolymer cements. Constr Build Mater 25:2805–2812. https://doi.org/10.1016/j.conbuildmat.2010.12.055
Heah CY, Kamarudin H, Al Bakri AM, Luqman M, Nizar IK, Liew YM (2011) Potential application of kaolin without Calcine as greener concrete : a review. Aust J Basic Appl Sci 5:1026–1035
B. Braggs, J. Ralston, R.S.C. Smart, Surface modification of kaolinite, WO96/017021, 1996
Ming H, Spark KM, Smart RSC (2001) Comparison of radio-frequency-plasma- and ion-beam-induced surface modification of kaolinite. J Phys Chem B 105:3196–3203. https://doi.org/10.1021/jp0031496
Tamo BS, Kamgang-Youbi G, Acayanka E, Simo LM, Tiya-Djowe A, Kuete-Saa D, Laminsi S, Tchadjie L (2016) Plasma chemical functionalisation of a cameroonian kaolinite clay for a greater hydrophilicity. Plasma Chem. Plasma Process. 36. https://doi.org/10.1007/s11090-016-9731-4
Sop-Tamo B, Acayanka E, Boyom-Tatchemo WF, Nzali S, Kamgang-Youbi G, Laminsi S (2018) Gliding arc plasma pre-treatment of kaolin in spatial post-discharge mode for removal of reactive red 2 dye from aqueous solution. Water Sci Technol 78:1448–1458. https://doi.org/10.2166/wst.2018.419
2005 EN196–1, Methods of testing cement - Part 1: Determination of strength. Eur Stand (2005) 1–33
Rollet AP, Bouaziz R (1972) L’analyse thermique: l’examen du processus chimique. Gauthier-Villars
A.B.B. Mohd Mustafa, Y.M. Liew, C.Y. Heah, F.M.T. Muhammad (2018) Clay-based materials in Geopolymer technology, in: Cem. Based Mater., Intech Open, p. 279. https://doi.org/10.5772/intechopen.74438
A.A.& R.O.O. U.O.Aroke (2013) Fourier-transform infrared characterization of kaolin,Granite,Bentonite and barite. J Chem Inf Model 53:1689–1699. https://doi.org/10.1017/CBO9781107415324.004
Liew YM, Kamarudin H, Al Bakri AMM, Luqman M, Khairul IN, Heah CY (2011) Investigating the possibility of utilization of kaolin and the potential of metakaolin to produce green cement for construction purposes – a review. Aust J Basic Appl Sci 5:441–449
Rees CA, Provis JL, Lukey GC, Van Deventer JSJ (2007) In situ ATR-FTIR study of the early stages of fly ash geopolymer gel formation. Langmuir. 23:9076–9082. https://doi.org/10.1021/la701185g
Tchadjié LN, Ekolu SO, Quainoo H, Tematio P (2021) Incorporation of activated bauxite to enhance engineering properties and microstructure of volcanic ash Geopolymer mortar composites. J Build Eng 41:102384. https://doi.org/10.1016/j.jobe.2021.102384
Rovnaník P (2010) Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr Build Mater 24:1176–1183. https://doi.org/10.1016/j.conbuildmat.2009.12.023
Yousef RI, El-eswed B, Alshaaer M, Khalili F, Rahier H (2012) Degree of reactivity of two kaolinitic minerals in alkali solution using zeolitic tuff or silica sand filler. Ceram Int 38:5061–5067. https://doi.org/10.1016/j.ceramint.2012.03.008
Prud E, Michaud P, Joussein E, Rossignol S (2012) Influence of raw materials and potassium and silicon concentrations on the formation of a zeolite phase in a geopolymer network during thermal treatment. J Non-Cryst Solids 358:1908–1916. https://doi.org/10.1016/j.jnoncrysol.2012.05.043
Zheng Z, Ma X, Zhang Z, Li Y (2019) In-situ transition of amorphous gels to Na-P1 zeolite in geopolymer: mechanical and adsorption properties. Constr Build Mater 202:851–860. https://doi.org/10.1016/j.conbuildmat.2019.01.067
Tchakoute HK, Rüscher CH, Djobo JNY, Kenne BBD, Njopwouo D (2015) Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl Clay Sci 107:1–7. https://doi.org/10.1016/j.clay.2015.01.023
Hounsi AD, Lecomte-nana GL, Djétéli G, Blanchart P (2013) Kaolin-based geopolymers : effect of mechanical activation and curing process. Constr Build Mater 42:105–113. https://doi.org/10.1016/j.conbuildmat.2012.12.069
Kenne BBD, Elimbi A, Cyr M, Manga JD, Kouamo HT (2015) Effect of the rate of calcination of kaolin on the properties of metakaolin-based geopolymers. J Asian Ceram Soc 3:130–138. https://doi.org/10.1016/j.jascer.2014.12.003
C.Y. Heah, H. Kamarudin, A.M. Mustafa Al Bakri, M. Binhussain, M. Luqman, I. Khairul Nizar, C.M. Ruzaidi, Y.M. Liew, Effect of curing profile on kaolin-based geopolymers, Phys. Procedia. 22 (2011) 305–311. https://doi.org/10.1016/j.phpro.2011.11.048
J.G.S. Van Jaarsveld, J.S.J. Van Deventer, A. Schwartzman, The potential use of geopolymeric materials to immobilise toxic metals: Part II. Material and leaching characteristics, Miner. Eng. 12 (1999) 75–91. https://doi.org/10.1016/S0892-6875(98)00121-6
Van Jaarsveld JGS, van Deventer JSJ, Lukey GC (2003) The characterisation of source materials in fly ash-based geopolymers. Mater Lett 57:1272–1280
B.D. Adkins, B.H. Davis, Comparison of nitrogen adsorption and mercury penetration results I. Pore volume and surface area obtained for type IV isotherms, Adsorpt. Sci. Technol. 5 (1988) 76–93. https://doi.org/10.1177/026361748800500108
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas / solid systems with special reference to the determination of surface area and porosity (recommendations 1984 ). Pure Appl Chem 57:603–619
Luna-Galiano Y, Fernández-Pereira C, Izquierdo M (2016) Contributions to the study of porosity in fly ash-based geopolymers. Relationship between degree of reaction , porosity and compressive strength. Mater Construcción 66:e098. https://doi.org/10.3989/mc.2016.10215
Yao X, Zhang Z, Zhu H, Chen Y (2009) Geopolymerization process of alkali – metakaolinite characterized by isothermal calorimetry. Thermochim Acta 493:49–54. https://doi.org/10.1016/j.tca.2009.04.002
Acknowledgements
The authors are grateful to Professor Elimbi Antoine of the University of Yaoundé I (Cameroon) for providing clay samples.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
B. Sop-Tamo: study conception and design, acquisition of data, analysis and interpretation of data, drafting of manuscript, L.N. Tchadjie: methodology, analysis and interpretation of data,writing - review & editing, J.B. Tarkwa: methodology, writing - review & editing, Thamer Alomayri: writing - review & editing, Hasan Assaedi: Writing - review & editing, J. P. Kamseu Mogo: writing - review & editing, J. Baenla: methodology, writing - review & editing, Kamgang-Youbi: writing - review & editing.
Corresponding author
Ethics declarations
This manuscript has not been published elsewhere in any form or language and has not been submitted to more than one journal for simultaneous consideration.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Declaration on Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
The gliding arc plasma treatment affects the geopolymeric reactivity of kaolin.
The gliding arc plasma-treated in spatial post-discharge mode is preferred to gliding arc plasma treatment in direct mode.
kaolin treated in spatial post-discharge mode gives the highest compressive strength.
Hydroxysodalite is the main crystalline phase after the geopolymerization process.
Rights and permissions
About this article
Cite this article
Sop-Tamo, B., Tchadjié, L.N., Tarkwa, J.B. et al. Effects of Non-thermal Plasma Treatment on the Geopolymerization of Kaolin Clay. Silicon 14, 3641–3652 (2022). https://doi.org/10.1007/s12633-021-01134-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-021-01134-z