Abstract
Purpose
Early postoperative mobilization can be hindered by orthostatic intolerance (OI). Postoperative OI has multifactorial pathogenesis, possibly involving both postoperative hypovolemia and autonomic dysfunction. We aimed to investigate the effect of mild acute blood loss from blood donation simulating postoperative hypovolemia, on both autonomic function and OI, thus eliminating confounding perioperative factors such as inflammation, residual anesthesia, pain, and opioids.
Methods
This prospective observational cohort study included 26 blood donors. Continuous electrocardiogram data were collected during mobilization and night sleep, both before and after blood donation. A Valsalva maneuver and a standardized mobilization procedure were performed immediately before and after blood donation, during which cardiovascular and tissue oxygenation variables were continuously measured by LiDCOrapid™ and Massimo Root™, respectively. The incidence of OI, hemodynamic responses during mobilization and Valsalva maneuver, as well as heart rate variability (HRV) responses during mobilization and sleep were compared before and 15 min after blood donation.
Results
Prior to blood donation, no donors experienced OI during mobilization. After blood donation, 6/26 (23%; 95% CI, 9 to 44) donors experienced at least one OI symptom. Three out of 26 donors (12%; 95% CI, 2 to 30) terminated the mobilization procedure prematurely because of severe OI symptoms. Cardiovascular and cerebral tissue oxygenation responses were reduced in patients with severe OI. After blood loss, HRV indices of total autonomic power remained unchanged but increased sympathetic and decreased parasympathetic outflow was observed during mobilization, but also during sleep, indicating a prolonged autonomic effect of hypovolemia.
Conclusion
We describe a specific hypovolemic component of postoperative OI, independent of postoperative autonomic dysfunction, inflammation, opioids, and pain.
Study registration
ClinicalTrials.gov (NCT04499664); registered 5 August 2020.
Résumé
Objectif
La mobilisation postopératoire précoce peut être entravée par une intolérance orthostatique (IO). L’IO postopératoire a une pathogenèse multifactorielle, impliquant peut-être à la fois une hypovolémie postopératoire et un dysfonctionnement autonome. Notre objectif était d’étudier l’effet d’une légère perte de sang aiguë due au don de sang simulant une hypovolémie postopératoire, à la fois sur la fonction autonome et sur l’IO, éliminant ainsi les facteurs périopératoires confondants tels que l’inflammation, l’anesthésie résiduelle, la douleur et les opioïdes.
Méthode
Cette étude de cohorte observationnelle prospective comprenait 26 personnes ayant donné leur sang. Des données d’électrocardiogramme continu ont été recueillies pendant la mobilisation et le sommeil nocturne, avant et après le don de sang. Une manœuvre de Valsalva et une procédure de mobilisation standardisée ont été réalisées immédiatement avant et après le don de sang, au cours desquelles les variables d’oxygénation cardiovasculaire et tissulaire ont été mesurées en continu avec les moniteurs LiDCOrapid™ et Massimo Root™, respectivement. L’incidence d’IO, les réponses hémodynamiques pendant la mobilisation et la manœuvre de Valsalva, ainsi que les réponses de variabilité de la fréquence cardiaque (VFC) pendant la mobilisation et le sommeil ont été comparées avant et 15 minutes après le don de sang.
Résultats
Avant le don de sang, aucune personne ayant fait un don de sang n’a ressenti d’IO pendant la mobilisation. Après le don de sang, 6/26 (23 %; IC 95 %, 9 à 44) des donneurs et donneuses ont manifesté au moins un symptôme d’IO. Trois personnes sur 26 (12 %; IC 95 %, 2 à 30) ont interrompu prématurément la procédure de mobilisation en raison de symptômes graves d’IO. Les réponses d’oxygénation des tissus cardiovasculaires et cérébraux ont été réduites chez les personnes atteintes d’IO sévère. Après la perte de sang, les indices de VFC de la puissance totale autonome sont demeurés inchangés, mais une augmentation du flux sympathique et une diminution du flux parasympathique ont été observées pendant la mobilisation, mais également pendant le sommeil, indiquant un effet autonome prolongé de l’hypovolémie.
Conclusion
Nous décrivons une composante spécifique hypovolémique de l’IO postopératoire, indépendante du dysfonctionnement autonome postopératoire, de l’inflammation, des opioïdes et de la douleur.
Enregistrement de l’étude
www.ClinicalTrials.gov (NCT04499664); enregistrée le 5 août 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Early postoperative mobilization is vital for rapid postoperative functional recovery and is a cornerstone in enhanced recovery after surgery.1,2 Nevertheless, it can be hindered by postoperative orthostatic intolerance (OI), characterized by subjective symptoms of dizziness, nausea, vomiting, visual disturbances, feeling of heat, presyncope, or even syncope.3 Numerous prospective studies have reported that early OI is a common clinical problem with 40–60% incidence after major elective surgery.4,5,6,7,8,9 The precise pathophysiologic mechanisms underlying postoperative OI are not yet fully clarified, but impaired orthostatic cardiovascular control and cerebral hypoperfusion due to autonomic dysfunction have been proposed.4,5,10,11
Autonomic nervous system function can be assessed in a simple and noninvasive manner using heart rate variability (HRV). Heart rate variability is the beat-to-beat variability in heart rate and reflects the relative balance between sympathetic and parasympathetic outflow.12,13 Postoperative autonomic dysfunction with limited autonomic capacity and decreased sympathetic and parasympathetic outflow characterized by decreased HRV indices is a well-described pathophysiologic phenomenon after various major surgeries and is linked to postoperative morbidity and mortality.10,14,15,16,17,18,19,20
Besides potentially inadequate autonomic cardiovascular control, postoperative patients might also be vulnerable to developing OI due to hypovolemia, which aggravates the postural reduction in central blood volume during mobilization. Postoperative hypovolemia is difficult to assess and might be present despite standardized perioperative fluid replacement therapy, due to preoperative fasting, diuresis, residual anesthesia effects, third spacing, and intraoperative and hidden blood loss.21 Furthermore, adequate perioperative fluid resuscitation is still a challenge in everyday clinical practice,22 and symptoms of postoperative OI might be the first clinical sign of postoperative hypovolemia.
Previous studies have addressed syncope,23,24 autonomic function,25,26 and basic hemodynamic changes during mobilization27 after blood donation. Nevertheless, changes in autonomic function over time and detailed changes in hemodynamic and tissue oxygenation variables have not yet been described in detail.
The current study aimed to investigate the isolated effect of mild hypovolemia on autonomic function over time and on the development of OI. Mild postoperative hypovolemia was simulated by blood donation of 450 mL in nonsurgical patients, thereby excluding perioperative confounding factors such as inflammation, residual anesthesia, pain, and opioid use. The primary outcome was change in parasympathetic autonomic function during mobilization after acute blood loss. Secondary outcomes were incidence of OI and severe OI; and changes in autonomic, hemodynamic, and tissue oxygenation responses during mobilization after acute blood loss.
Methods
This study was a prospective observational single-centre study performed at the Blood Bank at Copenhagen University Hospital Hvidovre. Twenty-six volunteers eligible for blood donation under the Danish health legislation were included between July and October 2020. Inclusion criteria were male gender, age 30–45 yr, ability to speak and understand Danish, and informed oral and written consent. Female donors and donors < 30 yr and > 45 yr were not included to avoid influence of hormonal changes and age, respectively, on the function of the autonomic nervous system. Exclusion criteria were previous history of OI and/or hypotension and use of antihypertensive medication. The trial was approved by the local ethics committee (H-19069845) and registered with the Danish data protection agency and at ClinicalTrials.gov (NCT04499664; 5 August 2020).
We used ePatch® (Delta Danish Electronics, Hørsholm, Denmark)28 for continuous electrocardiogram (ECG) monitoring and HRV data collection. The ePatch sensor was attached to the skin over the sternum 24 hr prior to blood donation and removed 24 hr afterwards.
Hemodynamic measurements were conducted in a quiet environment at the blood bank. Donors were not allowed to drink during the procedure and were seated in a semirecumbent position in a blood donor chair. After a resting period of five minutes, donors were instructed to perform a Valsalva maneuver (VM) by taking a deep inspiration and subsequently exhaling in a modified positive expiratory pressure device against a pressure of 40 mm Hg measured by a manometer. After a practice run, the maneuver was repeated twice, separated by one-minute rest. The curve with the best physiologic response, evaluated by the highest overshoot in phase IV by visual inspection was used for analysis. The Valsalva maneuver allows dynamic assessment of autonomic activity and can be used to diagnose a variety of abnormal responses.29,30
Immediately following VM, donors were instructed to perform a standardized mobilization procedure including semirecumbent rest (five minutes), standing still (five minutes), and finally semirecumbent rest again (five minutes). Mobilization was terminated prematurely in any position if patients experienced unbearable symptoms of OI or upon a decrease of systolic arterial pressure (SAP) > 30 mm Hg. The mobilization procedure was followed by venipuncture and withdrawal of approximately 450 mL of blood over 10–15 min. After 15 min, the procedure was repeated as previously described. During mobilization, continuous arterial blood pressure was measured by finger cuffs applied on the second and third finger of each hand at heart level by using LiDCOrapid™ (LiDCO Ltd., London, UK) and CNAP® Monitor (CNSystems, Graz, Austria), respectively. These provide precise and real-time estimates of arterial blood pressure, comparable with invasive intra-arterial systems during hemodynamic perturbations.31 Muscle and cerebral oxygenation were recorded with two-second intervals using Masimo Root® near-infrared spectroscopy with optodes placed on the biceps brachii muscle and the forehead. The perfusion index (PI)32 was measured using a Masimo Root® Radical 7 Pulse CO-Oximeter® (Masimo, Irvine, CA, USA). The ECG was recorded continuously.
Orthostatic classification
During the mobilization procedure, patients were classified as having OI if they experienced dizziness, nausea, blurred vision, a feeling of heat, or presyncope during standing, regardless of blood pressure. Symptoms were assessed using a standardized questionnaire and were graded as bearable or unbearable. Donors not being able to complete the mobilization procedure because of unbearable OI symptoms were classified as having severe OI, regardless of blood pressure. Orthostatic hypotension was defined as a decrease in SAP of ≥ 20 mm Hg or diastolic arterial pressure (DAP) ≥ 10 mm Hg during sitting or standing when compared with supine prior to mobilization.3
Data analysis
The ePatch HRV data were visually inspected for artifacts and analyzed using Cardiscope™ software (SMART Medical, Gloucestershire, UK). Heart rate variability mobilization data were obtained for the five-minute periods of semirecumbent and standing positions, while HRV sleep data were obtained for the best six hours of night sleep before and after blood donation, which was visually identified by the highest activity in the high frequency (HF) band. The following HRV variables were extracted: 1) HRV time-domain measures—standard deviation of NN-intervals (SDNN), % of successive NN intervals that differ more than 50% (pNN50%), root mean square of successive NN-interval differences (rMSSD); 2) HRV frequency domain measures—the absolute power of low (0.04–0.15 Hz) and high (0.15–0.4 Hz) frequency band (LF-i and HF-i, respectively), the relative power of low (0.04–0.15 Hz) and high (0.15–0.4 Hz) frequency band in % (LF-i norm and HF-i norm, respectively), the LF-i/HF-I ratio, and the absolute total HRV power (TP-i); and 3) HRV nonlinear measurements—Poincaré plot standard deviation perpendicular and along the line of identity (SD1 and SD2, respectively) and SD1/SD2 ratio. SDNN and TP-i are estimates of overall HRV and total power of the autonomic nervous system and reflect both sympathetic and parasympathetic functions. The HF band is almost exclusively affected by parasympathetic afferents, while the LF band is known to primarily relate to sympathetic activity, but also receives parasympathetic modulation. The normalized LF and HF values represent the relative value of each power component in proportion to the total power. The LF/HF ratio is used as a metric of sympathetic-parasympathetic balance. The rMMSD is considered to be the most precise marker for parasympathetic modulation of the heart.12
The finger arterial pressure curve during VM was analyzed using LabChart® Pro software (ADInstruments, Dunedin, New Zealand). Visual parameters of VM were composed of four hemodynamic hallmarks following baseline: 1) “valley,” where SAP decline stops in early phase II; 2) “rise,” where SAP incline ceases in late phase II; 3) “recovery,” where SAP returns to baseline in phase IV, and 4) “overshoot”, where SAP rises from baseline to maximal value in phase IV30 (Fig. 1).
The finger arterial pressure curve and derived cardiovascular values from the mobilization procedure were analyzed with LiDCOviewPro version 1.1 software (LiDCO Ltd., London, UK). Each curve was visually inspected for artifacts before averaging and such data were excluded. During the semirecumbent rest period, values were averaged over five minutes while during standing, values were averaged over the last ten seconds before termination of each posture, both in patients completing the mobilization procedure and terminating the mobilization procedure prematurely.
Outcomes
The primary outcome was a change in the parasympathetic output (rMSSD) during mobilization after acute blood loss. Secondary outcomes were incidence of OI and severe OI during mobilization before and after blood donation, changes in HRV variables representing total autonomic power (TP, SDNN), sympathetic (LF-i, LF-i norm, LF-i/HF-i) and parasympathetic (rMSSD, pNN50%, HF-i, HF-i norm, SD1) output during mobilization and sleep, changes in VM visual responses, and changes in hemodynamic and tissue oxygenation variables during mobilization and before and after blood donation.
Statistical analysis
All data were evaluated for normal distribution by Q-Q plots and histograms before analysis. Normally and nonnormally distributed continuous variables are presented as mean (standard deviation [SD]), and median [interquartile range (IQR)], respectively. Categorical variables are reported as the frequency with percentages. Differences in predonation and postdonation variables and in semirecumbent and standing values were identified using the paired t test or the Wilcoxon rank-sum test. Differences in postdonation values between orthostatic tolerant, orthostatic intolerant, and severe orthostatic intolerant patients were identified using an unpaired t test or Mann–Whitney test. Statistical analysis was carried out in IBM SPSS for Windows version 25 (IBM Corp., Armonk, NY, USA). A two-sided P < 0.05 was considered statistically significant.
Sample size calculation
No formal power analysis was performed since this was an explorative hypothesizing observational study and no data on overall changes in autonomic parameters over time after induced hypovolemia were available.
Results
Twenty-six male blood donors with a mean (SD) age of 37 (5) yr, height of 182 (8) cm, and weight of 90 (17) kg were included in the study.
Because of frequent premature atrial complexes, HRV data during mobilization and sleep from one donor were excluded from the analysis. Heart rate variability data on sleep were missing for one donor because of a disconnected ePatch electrode the night before blood donation. Because of trace artifacts, LiDCO data after blood donation were missing from three donors. In two of these, SAP, DAP, mean arterial pressure (MAP), and heart rate (HR) data were extracted from the CNAP monitor while stroke volume (SV), cardiac output (CO), and systemic vascular resistance (SVR) data were still missing. Due to artifacts both in LiDCO and CNAP data, the latter donor (with severe OI) only had HR data available. In donors that terminated mobilization prematurely because of intolerable symptoms, HRV data were extracted only while the heart rate was stable. Complete HRV and hemodynamics data were obtained and analyzed for 24 and 23 blood donors, respectively.
Heart rate variability
Absolute HRV data during mobilization (n = 25) are presented in Table 1. There were no significant differences in SDNN and TP-i in semirecumbent positions before and after blood donation. MeanNN, rMSSD, pNN50, HF-i, HF-i norm, SD1, and SD1/SD2 were significantly reduced from semirecumbent to standing position both before and after blood donation, while LF-i norm and LF-i/HF-i were significantly increased. In the semirecumbent position, rMSSD, HF-I, HF-I norm, and SD1 significantly decreased from before to after blood donation, while LF-I norm and LF-i/HF-I were significantly increased. In the standing position, TP-i and LF-i/HF-i was increased from before to after blood donation, while meanNN, rMSSD, pNN50, SD1 and SD1/SD2 were decreased.
Relative changes in HRV variables from supine to standing before and after blood donation are presented in Table 2. There were no significant changes apart from significantly increased TE-I and LF-i and significantly decreased HF-i.
Heart rate variability data during sleep (n = 24) are presented in Table 3.
There were no significant differences in SDNN and TP-i before and after blood donation. The following markers were significantly reduced during sleep from before to after blood donation: rMSSD, pNN50, HF-i, HFi-norm, SD1, and SD1/SD2, while LF-i norm and LF-i/HF-i were significantly increased.
Valsalva maneuver
Before blood donation, all donors had a normal physiologic response to VM with identifiable rise, recovery, and overshoot. After blood donation, all donors expect one had a normal physiologic response. A single donor presented with a visually abnormal VM curve (Fig. 2), typical of neurogenic autonomic dysfunction characterized by a missing rise, recovery, and overshoot. The same donor could not complete the mobilization procedure after VM because of severe OI symptoms, as discussed below.
Mobilization procedure
Absolute hemodynamic and tissue oxygenation values during mobilization are presented in Table 4.
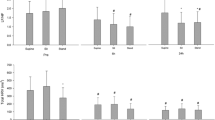
Relative hemodynamic and tissue oxygenation values during mobilization in orthostatic tolerant and intolerant donors, before and after blood donation, are presented in Fig. 3.
Changes in hemodynamic variables pre and post blood donation in orthostatic tolerant donors (OT) and orthostatic intolerant donors with severe symptoms (OI-SEV) and without severe symptoms (OI-NONSEV) during a standardized mobilization procedure. All data presented as mean ± SEM: *P < 0.05 compared with OT patents after blood donation.
CO = cardiac output; DAP = diastolic arterial pressure; HR = heart rate; MAP = mean arterial pressure; PPI = peripheral perfusion index; SAP = systolic arterial pressure; ScO2 = cerebral tissue oxygenation; SEM = standard error of the mean; SmO2 = muscular tissue oxygenation; SV = stroke volume; SVR = systemic vascular resistance
No donors experienced OI or orthostatic hypotension during mobilization before blood donation. After blood donation, 6/26 donors (23%; 95% CI, 9 to 44) experienced OI symptoms, three of whom completed the mobilization procedure and three of whom had severe OI and were thus unable to complete the mobilization procedure. The three donors (12%; 95% CI, 2 to 30) who completed the mobilization procedure experienced a single OI symptom: dizziness, visual disturbance, and a feeling of heat, respectively. They did not present with concomitant fall in SAP, DAP, MAP, HR, CO, or ScO2 during mobilization when compared with orthostatic tolerant donors (Fig. 3). When compared with orthostatic tolerant donors, the 3/26 (12%; 95% CI, 2 to 30) donors with severe OI had statistically significant changes in the following hemodynamic variables from the semirecumbent to standing position: ΔSAP (2 [8] vs −36 mm Hg; difference in means [MD], −38 mm Hg; 95% CI, −25 to −51; P = 0.009), ΔDAP (14 [6] vs −25 mm Hg; MD, −39 mm Hg; 95% CI, −28 to −50; P = 0.009), ΔMAP (8 [6] vs −31 mm Hg; MD, −40 mm Hg; 95% CI, −30 to −50; P = 0.009), ΔHR (31 [13] vs −1 beats·min−1; MD, −28 beats·min−1; 95% CI, −11 to −50; P = 0.002), and ΔScO2 (−2 [2]% vs −10; MD, −6%; 95% CI, −3 to −9; P = 0.002) (Fig. 3). No significant changes in were observed in ΔSV, ΔCO, ΔSVR, ΔPPI, or ΔScmO2 between orthostatic tolerant and intolerant patients.
All three severe OI donors experienced at least dizziness. Two presented with vasovagal presyncope (Fig. 4), while the latter presented with neurogenic autonomic dysfunction that was already apparent during VM prior to mobilization, as already discussed earlier (Fig. 2).
Discussion
This single-centre prospective observational study suggests a specific hypovolemic component in development of postoperative OI, independent of other postoperative factors such as autonomic dysfunction characterized by decreased HRV indices, inflammation, residual anesthesia, pain, and opioids. Heart rate variability analysis during mobilization and sleep after blood donation revealed increased sympathetic and decreased parasympathetic outflow, suggesting a relevant physiologic response to hypovolemia, which persisted over time. We also describe that mild hypovolemia induced by 450 mL unreplaced blood loss leads to OI in 23% (95% CI, 9 to 44) and severe OI in 12% (95% CI, 2 to 30) of cases.
Postoperative autonomic dysfunction might contribute to the development of OI, hindering early postoperative mobilization.4,5,11 The etiology of postoperative autonomic dysfunction is multifactorial and whether the postoperative patient develops symptoms of OI likely depends on the severity and interplay of contributing factors such as age, comorbidities, medication, surgical stress-response, residual anesthesia, postoperative use of opioids, hypovolemia, pain during mobilization, and the patient’s autonomic coping capacity.
Heart rate variability is the beat-to-beat variability in heart rate, a sensitive marker of altered autonomic function, that is reduced under different stress conditions.12,13 Many studies have reported decreased HRV indices after abdominal,14,15,18 orthopedic,10 and thoracic surgery,17,19 demonstrating impaired autonomic regulation in the early postoperative period. Contrary to these studies, we found no significant changes during sleep in HRV indices of total autonomic power (SDNN, TP-i) after acute mild blood loss, showing an intact autonomic cardiovascular regulation and autonomic capacity, when blood loss is not associated with surgical stress. Additionally, we found increased sympathetic (LF-i, LF-i norm, LF-i/HF-i) and decreased parasympathetic (rMSSD, pNN50, HF-i, HFi-norm, SD1) activity after blood donation, which persisted during sleep the first night post donation, indicating a relevant and prolonged physiologic response to hypovolemia, contrary to suppressed sympathetic and parasympathetic response, previously described after surgery with comparable blood loss.4,18 As HRV is affected by the sleep-wake cycle and activity, we extracted and compared E-patch® HRV data during sleep before and after blood donation.
The only previous study evaluating HRV changes during postoperative mobilization reported a paradoxical postoperative HRV shift towards the parasympathetic HF range when going from a supine to standing position.10 Contrary to this, our study describes a normal physiologic response to postural change with increased LF/HF ratio due to increased sympathetic and decreased parasympathetic outflow, also in line with previous studies examining normal physiology in nonsurgical patients.33,34 Our findings might therefore suggest that the surgical stress response and/or other perioperative factors—but not mild blood loss as an isolated factor—contribute to postoperative autonomic dysfunction, characterized by decreased HRV indices.
Nevertheless, autonomic dysfunction is a heterogeneous phenomenon. Even though we assume normal autonomic function for the entire cohort as measured by HRV, two donors with severe OI presented with postdonation vasovagal presyncope, a type of reflex autonomic dysfunction characterized by paradoxical sympathetic withdrawal common in young healthy individuals due to hypersensitivity.35 This pathophysiologic mechanism differs profoundly from neurogenic autonomic hyporesponsiveness,30 apparent during VM (Fig. 3) in the latter donor experiencing severe OI. Hence, acute hypovolemia per se might induce autonomic dysfunction of different types, depending on the physiology of the individual patient.
We aimed to model postoperative hypovolemia by acute blood loss in healthy young male blood donors. There are several limitations to this model. Firstly, fluid therapy is used to replace perioperative fluid and blood loss from the surgical field and wound drainage. Nevertheless, estimating patients’ perioperative fluid deficit is inexact because of capillary leak,36 hidden intra- and postoperative blood loss,21,37 and vasoplegia due to residual anesthesia,38 among other factors. Clinical hypovolemia may therefore be present and clinically unrecognized, which underlines the importance of examining its isolated contribution to the development of OI. Secondly, the surgical population is heterogeneous, including the elderly and comorbid. Nevertheless, if hypovolemically induced OI symptoms are present in the young and healthy, it is likely that they will also occur in postoperative, elderly, and comorbid patients.
This is to our knowledge the first detailed study to isolate and investigate the effect of mild hypovolemia on autonomic function over time. Further strengths of our study include standardized blood donation, mobilization, and symptom questionnaires. Performing VM before mobilization might have confounded hemodynamic responses during mobilization; nevertheless, this is unlikely since the rest period in the semirecumbent position should have replenished neurotransmitter stores. We did not measure inflammatory markers before and after blood donation; however, all donors fulfilled the blood donation criteria and had no ongoing symptoms of inflammation.
Conclusion
This single-centre prospective observational study suggests that acute mild blood loss, as an isolated factor, does not contribute to postoperative autonomic dysfunction, as assessed by HRV indices. Nevertheless, we suggest a specific hypovolemic component in the development of postoperative OI.
References
Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997; 78: 606–17. https://doi.org/10.1093/bja/78.5.606
Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008; 248: 189–98. https://doi.org/10.1097/SLA.0b013e31817f2c1a
Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011; 21: 69–72. https://doi.org/10.1007/s10286-011-0119-5
Jans Ø, Bundgaard-Nielsen M, Solgaard S, Johansson PI, Khelet H. Orthostatic intolerance during early mobilization after fast-track hip arthroplasty. Br J Anaesth 2012; 108: 436–43. https://doi.org/10.1093/bja/aer403
Bundgaard-Nielsen M, Jørgensen CC, Jørgensen TB, Ruhnau B, Secher NH, Kehlet H. Orthostatic intolerance and the cardiovascular response to early postoperative mobilization. Br J Anaesth 2009; 102: 756–62. https://doi.org/10.1093/bja/aep083
Eriksen JR, Munk-Madsen P, Kehlet H, Gögenur I. Orthostatic intolerance in enhanced recovery laparoscopic colorectal resection. Acta Anaesthesiol Scand 2019; 63: 171–7. https://doi.org/10.1111/aas.13238
Lindberg-Larsen V, Petersen PB, Jans Ø, Beck T, Khelet H. Effect of pre-operative methylprednisolone on orthostatic hypotension during early mobilization after total hip arthroplasty. Acta Anaesthesiol Scand 2018; 62: 882–92. https://doi.org/10.1111/aas.13108
Bundgaard-Nielsen M, Jans Ø, Müller RG, et al. Does goal-directed fluid therapy affect postoperative orthostatic intolerance? A randomized trial. Anesthesiology 2013; 119: 813–23. https://doi.org/10.1097/ALN.0b013e31829ce4ea
Jans Ø, Mehlsen J, Kjærsgaard-Andersen P, et al. Oral midodrine hydrochloride for prevention of orthostatic hypotension during early mobilization after hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Anesthesiology 2015; 123: 1292–300. https://doi.org/10.1097/ALN.0000000000000890
Jans Ø, Brinth L, Kehlet H, Mehlsen J. Decreased heart rate variability responses during early postoperative mobilization - an observational study. BMC Anesthesiol 2015; 15: 120. https://doi.org/10.1186/s12871-015-0099-4
Jans Ø, Kehlet H. Postoperative orthostatic intolerance: a common perioperative problem with few available solutions. Can J Anesth 2017; 64: 10–5. https://doi.org/10.1007/s12630-016-0734-7
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability, standards of measurement, physiologic interpretation, and clinical use. Circulation 1996; 93: 1043–65. https://doi.org/10.1161/01.CIR.93.5.1043
Ernst G. Heart-rate variability—more than heart beats? Front Public Health 2017; 5: 240. https://doi.org/10.3389/fpubh.2017.00240
Cheng W, Liu J, Zhi M, et al. Stress and autonomic nerve dysfunction monitoring in perioperative gastric cancer patients using a smart device. Ann Noninvasive Electrocardiol 2022; 27: e12903. https://doi.org/10.1111/anec.12903
Haase O, Langelotz C, Scharfenberg M, Schwenk W, Tsilimparis N. Reduction of heart rate variability after colorectal resections. Langenbecks Arch Surg 2012; 397: 793–9. https://doi.org/10.1007/s00423-012-0903-2
Ireland N, Meagher J, Sleigh JW, Henderson JD. Heart rate variability in patients recovering from general anesthesia. Br J Anaesth 1996; 76: 657–62. https://doi.org/10.1093/bja/76.5.657
Komatsu T, Kimura T, Nishiwaki K, Fujiwara Y, Sawada K, Shimada Y. Recovery of heart rate variability profile in patients after coronary artery surgery. Anesth Analg 1997; 85: 713–8. https://doi.org/10.1097/00000539-199710000-00001
Ushiyama T, Nakatsu T, Yamane S, et al. Heart rate variability for evaluating surgical stress and development of postoperative complications. Clin Exp Hypertens 2008; 30: 45–55. https://doi.org/10.1080/10641960701813908
Kimura T, Komatsu T, Takezawa J, Shimada Y. Alterations in spectral characteristics of heart rate variability as a correlate of cardiac autonomic dysfunction after esophagectomy or pulmonary resection. Anesthesiology 1996; 84: 1068–76. https://doi.org/10.1097/00000542-199605000-00008
Frandsen MN, Mehlsen J, Foss NB, Kehlet H. Preoperative heart rate variability as a predictor of perioperative outcomes: a systematic review without meta-analysis. J Clin Monit Comput 2022; 36: 947–60. https://doi.org/10.1007/s10877-022-00819-z
Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty. Correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br 2004; 86: 561–5.
Miller TE, Myles PS. Perioperative fluid therapy for major surgery. Anesthesiology 2019; 130: 825–32. https://doi.org/10.1097/ALN.0000000000002603
Morand C, Coudurier N, Rolland C, et al. Prevention of syncopal-type reactions after whole blood donation: a cluster-randomized trial assessing hydration and muscle tension exercise. Transfusion 2016; 56: 2412–21. https://doi.org/10.1111/trf.13716
Trouern-Trend JJ, Cable RG, Badon SJ, Newman BH, Popovsky MA. A case-controlled multicenter study of vasovagal reactions in blood donors: influence of sex, age, donation status, weight, blood pressure, and pulse. Transfusion 1999; 39: 316–20. https://doi.org/10.1046/j.1537-2995.1999.39399219291.x
Paprika D, Judák L, Korsós A, Rudas L, Zöllei. The effects of acute blood loss on blood pressure recovery from the Valsalva maneuver. Auton Neurosci 2011; 160: 103–6. https://doi.org/10.1016/j.autneu.2010.11.008
Zöllei E, Paprika D, Makra P, Gingl Z, Vezendi K, Rudas L. Human autonomic responses to blood donation. Auton Neurosci 2004; 110: 114–20. https://doi.org/10.1016/j.autneu.2003.10.003
Klapper E, Pepkowitz SH, Czer L, Inducil C, Scott L, Goldfinger D. Confirmation of the safety of autologous blood donation by patients awaiting heart or lung transplantation. A controlled study using hemodynamic monitoring. J Thorac Cardiovasc Surg 1995; 110: 1594–9. https://doi.org/10.1016/S0022-5223(95)70018-8
Saadi DB, Sørensen HB, Hansen IB, Egstrip K, Jennum P, Hoppe K. ePatch ® - a clinical overview, 2014. Available from URL: https://backend.orbit.dtu.dk/ws/portalfiles/portal/102966188/ePatch_A_Clinical_Overview_DTU_Technical_Report.pdf (accessed February 2023).
Pstras L, Thomaseth K, Waniewski J, Balzani I, Bellavere F. The Valsalva manoeuvre: physiology and clinical examples. Acta Physiol 2016; 217: 103–19. https://doi.org/10.1111/apha.12639
Palamarchuk IS, Baker J, Kimpinski K. The utility of Valsalva maneuver in the diagnoses of orthostatic disorders. Am J Physiol Regul Integr Comp Physiol 2016; 310: R243–52. https://doi.org/10.1152/ajpregu.00290.2015
Jeleazcov C, Krajinovic L, Münster T, et al. Precision and accuracy of a new device (CNAP™) for continuous non-invasive arterial pressure monitoring: assessment during general anesthesia. Br J Anaesth 2010; 105: 264–72. https://doi.org/10.1093/bja/aeq143
Goldman JM, Petterson MT, Kopotic RJ, Barker SJ. Masimo signal extraction pulse oximetry. J Clin Monit Comput 2000; 16: 475–483. https://doi.org/10.1023/a:1011493521730
Montano N, Ruscone TG, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90: 1826–31. https://doi.org/10.1161/01.cir.90.4.1826
O’Leary DD, Kimmerly DS, Cechetto AD, Shoemaker JK. Differential effect of head-up tilt on cardiovagal and sympathetic baroreflex sensitivity in humans. Exp Physiol 2003; 88: 769–74. https://doi.org/10.1113/eph8802632
Grubb BP, Karabin B. Neurocardiogenic syncope. In: Aminoff MJ, Daroff RB (Eds.). Encyclopedia of the Neurologic Sciences, 2nd ed. New York: Elsevier; 2014: 367–75. https://doi.org/10.1016/B978-0-12-385157-4.01164-7
Strunden MS, Heckel K, Goetz AE, Reuter DA. Perioperative fluid and volume management: physiologic basis, tools and strategies. Ann Intensive Care 2011; 1: 2. https://doi.org/10.1186/2110-5820-1-2
Foss NB, Kehlet H. Hidden blood loss after surgery for hip fracture. J Bone Joint Surg Br 2006; 88: 1053–9. https://doi.org/10.1302/0301-620X.88B8.17534
Liu H, Yu L, Yang L, Green MS. Vasoplegic syndrome: an update on perioperative considerations. J Clin Anesth 2017; 40: 63–71. https://doi.org/10.1016/j.jclinane.2017.04.017
Author contributions
Anna-Marija Hristovska contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Bodil Uldall-Hansen and Louise B. Andersen contributed to the acquisition of data and drafting the article. Jesper Mehlsen, Henrik Kehlet, and Nicolai B. Foss contributed to study conception and design, interpretation of data, and drafting the article.
Acknowledgments
We would like to acknowledge our research nurse Mette Grentoft from the Department of Anesthesiology at Copenhagen University Hospital Hvidovre for her invaluable help during the hemodynamic measurement of the blood donors. We would also like to thank the personnel at the Blood Bank at Copenhagen University Hospital Hvidovre for their great help in recruiting blood donors.
Disclosures
No competing interests declared.
Funding statement
The work was supported by the CANDYS Foundation. Open access funding was provided by Royal Library, Copenhagen University Library.
Prior conference presentations
Data from this article were presented as a poster presentation at the Evidence Based Perioperative Medicine (EBPOM) World Congress 2022 (London, UK; 5–7 June 2022) and as an oral presentation at the Yearly Danish Meeting of Anesthesiology and Intensive Care in November 2022 in Copenhagen, Denmark.
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hristovska, AM., Uldall-Hansen, B., Mehlsen, J. et al. Orthostatic intolerance after acute mild hypovolemia: incidence, pathophysiologic hemodynamics, and heart-rate variability analysis—a prospective observational cohort study. Can J Anesth/J Can Anesth 70, 1587–1599 (2023). https://doi.org/10.1007/s12630-023-02556-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-023-02556-6