Abstract
Purpose
Postdischarge opioid prescriptions are reportedly much higher in Canada than in other countries. To assess potentially contributing factors, we examined trends after abdominal and orthopedic surgeries in British Columbia (BC).
Methods
Using the BC Ministry of Health’s databases on physician billings, hospital discharge abstracts, and medication dispensations in community pharmacies for the period 2003–2016, we assembled a cohort of 263,056 patients who received laparoscopic appendectomy (LA, 11%), laparoscopic cholecystectomy (LC, 30%), open inguinal or femoral hernia repair (IHR, 20%), total hip arthroplasty (THA, 18%), or total knee arthroplasty (TKA, 22%). Adjusting for covariates using generalized linear modeling, we measured trends in percentages of patients dispensed opioids postdischarge (opioid rate) within 30 days after surgery, by surgery type, opioid type, prior use, surgeon, and trends in morphine milligram equivalents of first dispensations (MME) with 95% confidence intervals (CI).
Results
Opioid dispensation rates rose steadily. Mean annual increases were 1.7% in LA; 1.3% in LC; 0.8% in IHR; 0.9% in THA; and 0.8% in TKA. By 2016, rates were 69% in LA; 76% in LC; 81% in IHR; 88% in THA; and 94% in TKA. Codeine dispensations fell 2.4% (abdominal) and 3.1% (orthopedic) per year while tramadol dispensations increased 3.6% (abdominal) and 1.7% (orthopedic). Hydromorphone dispensations increased 2.9% per year (orthopedic); oxycodone was level at 22% between 2007 and 2014, but then fell. The mean MME rose 8 mg⋅yr-1 (95% CI, 7 to 9) (abdominal) and 61 mg⋅yr-1 (95% CI, 58 to 64) (orthopedic). Variation in rates was greater among abdominal than orthopedic surgeons.
Conclusion
Rising opioid dispensation rates, together with shifts to prescribing higher MME opioids, doubled MME per patient in first dispensations postdischarge after abdominal or orthopedic surgery from 2003 to 2016 in BC.
Résumé
Objectif
Les ordonnances d’opioïdes après le congé seraient beaucoup plus élevées au Canada que dans d’autres pays. Afin d’évaluer les facteurs contributifs potentiels, nous avons examiné les tendances après les chirurgies abdominales et orthopédiques en Colombie-Britannique (C.-B.).
Méthode
En utilisant les bases de données du ministère de la Santé de la Colombie-Britannique de facturation des médecins, les résumés des congés d’hôpital et les délivrances de médicaments dans les pharmacies communautaires pour la période 2003-2016, nous avons regroupé une cohorte de 263 056 patients ayant bénéficié d’une appendicectomie par laparoscopie (AL, 11 %), d’une cholécystectomie par laparoscopie (CL, 30 %), d’une réparation ouverte de hernie inguinale ou fémorale (RHI, 20 %), d’une arthroplastie totale de la hanche (ATH, 18 %) ou d’une arthroplastie totale du genou (ATG, 22 %). En tenant compte des covariables à l’aide d’une modélisation linéaire généralisée, nous avons mesuré les tendances dans les pourcentages de patients ayant reçu des opioïdes après leur congé (taux de délivrance d’opioïdes) dans les 30 jours suivant leur chirurgie, par type de chirurgie, type d’opioïde, utilisation antérieure, chirurgien et tendances des équivalents de morphine en milligrammes (EMM) des premières délivrances avec des intervalles de confiance (IC) à 95 %.
Résultats
Les taux de délivrance d’opioïdes ont augmenté de manière constante. Les augmentations annuelles moyennes étaient de 1,7 % pour les AL, 1,3 % pour les CL, 0,8 % pour les RHI, 0,9 % pour les ATH, et 0,8 % pour les ATG. En 2016, les taux étaient de 69 % pour les AL, 76 % pour les CL, 81 % pour les RHI, 88 % pour les ATH, et 94 % pour les ATG. Les dispenses de codéine ont chuté de 2,4 % (chirurgie abdominale) et de 3,1 % (chirurgie orthopédique) par année, tandis que les délivrances de tramadol ont augmenté de 3,6 % (chirurgie abdominale) et de 1,7 % (chirurgie orthopédique). Les délivrances d’hydromorphone ont augmenté de 2,9 % par année (orthopédie); l’oxycodone était à 22 % entre 2007 et 2014, mais a ensuite diminué. Les EMM moyens ont augmenté de 8 mg·an-1 (IC 95 %, 7 à 9) (chirurgie abdominale) et de 61 mg·an-1 (IC 95 %, 58 à 64) (chirurgie orthopédique). La variation des taux était plus importante parmi les chirurgiens abdominaux que chez les chirurgiens orthopédistes.
Conclusion
L’augmentation des taux de délivrance d’opioïdes, ainsi que le passage à une prescription d’opioïdes plus élevés en EMM, ont doublé les EMM par patient dans les premières délivrances après leur congé après une chirurgie abdominale ou orthopédique de 2003 à 2016 en Colombie-Britannique.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Opioids play an important role in perioperative pain management. Nevertheless, a growing epidemic of opioid misuse has driven prescribers, health systems, and governments worldwide to scrutinize the rates and volumes of prescription opioids dispensed to patients in postoperative settings.1,2,3 According to a 2020 report from the International Narcotics Control Board, Canada ranks among the highest average global consumers of opioids for pain management.4 A recent retrospective study involving 223,834 patients in Ontario, the USA, and Sweden found rates of discharge opioid prescriptions after general orthopedic and abdominal surgeries were much higher in Ontario (79%) and the USA (76%) than in Sweden (11%).5 These findings are worrisome for Canadians as overprescription of opioids in the postsurgical setting has been associated with persistent postsurgical opioid use (continued dispensation of opioids beyond 90 days after surgery) in opioid-naïve patients.1,6–12
Abdominal and orthopedic surgeries are among the most common procedures in British Columbia (BC), with over 30,000 appendectomies, cholecystectomies, knee arthroplasties, and hip arthroplasties performed between 2019 and 2020.13 The estimated rates of persistent opioid use after these procedures vary, ranging from 1.5% after inguinal hernia repair to 4.3% after hip arthroplasty and over 5% after laparoscopic cholecystectomy (LC).8,9,14 This variability may partially stem from inherent differences in surgical procedure and patient-related factors; however, studies have found significant heterogeneity in postsurgical opioid prescription practices, even for identical procedures.11,12,14–16 Many opioid prescriptions are dispensed in far greater quantity and duration than would be typically required for postsurgical analgesia.10,15–17
The BC Ministry of Health (Ministry) continuously collects data from surgeons’ electronic billings and medications dispensed in all community pharmacies in BC, which potentially could enable low-cost, province-wide, real-time monitoring of trends and variations in postdischarge rates of opioid use as the opioid crisis evolves and the Enhanced Recovery After Surgery pathways are adopted.18 While showing feasibility of rapid monitoring, we had an opportunity to conduct a retrospective cohort analysis to explore historical trends by surgery type, opioid type, prior use, and surgeon; to compare our results with those from other jurisdictions; and to assess factors associated with high rates of opioid prescribing.
Methods
Data sources
We accessed Population Data BC’s repository of four Ministry databases:19,20,21,22 1) Medical Services Plan (MSP) physicians’ billing data, which are updated continuously, 2) hospital discharge abstract data, which are updated episodically and consolidated annually, 3) PharmaNet, which contains medication dispensing data from all community pharmacies in BC and is updated continuously, and 4) Client Registry, which contains the Ministry’s patient census, all linked by deidentified personal health numbers. For the Surgical Quality Outcomes Reports (SQOR) project, Population Data BC extracted the data on all patients who had any surgery in BC hospitals from 2002 to 2016.
Population Data BC were not an intermediary when we also analyzed the data for the SQOR Extension project directly using the same databases in the BC Ministry of Health’s Healthideas data warehouse, not only surgeries from 2003 to 2016 but also surgeries from 2017 to 2019. Data were not linkable between the SQOR and SQOR Extension projects, both of which were separately approved by The University of British Columbia’s Clinical Research Ethics Board (Vancouver, BC, Canada; #H14-01717 and #H20-00340).
Cohorts
Using MSP’s surgical procedure codes from surgeons’ billing data, we created five cohorts per year of patients age 18 and over who were eligible for MSP coverage of their surgery: laparoscopic appendectomy (LA), LC, open inguinal or femoral hernia repair (IHR), total hip arthroplasty (THA), and/or total knee arthroplasty (TKA) (Electronic Supplementary Material [ESM] eTable 1). The first three procedures were selected to compare 2016 rates of postdischarge opioid prescription with those reported in two recent international studies of postsurgical opioid prescribing rates in the USA, Sweden, and seven other countries.5,23 The orthopedic surgeries were chosen by the SQOR Extension project to show rapid monitoring. The patients were required also to have a Canadian Classification of Health Interventions code for the same procedure in the hospital data (ESM eTable 1). Patients were excluded if they had additional procedures besides the selected surgeries. If a patient had two surgeries of any one type within one calendar year, only the first was included. Cohorts assembled from the SQOR Extension project excluded patients with prior opioid dispensation or cancer diagnoses (ESM eTable 2) in the year before their surgery. To be consistent with the international studies,5,23 patients selected for the SQOR cohorts were not excluded if they had used opioids in the year before surgery but were later stratified by such use, or adjusted for it in multivariable modeling.
Opioid dispensing
By linking MSP billing data to PharmaNet by deidentified personal health number and surgery date in each calendar year, records of dispensations within 30 days after surgery were extracted if the chemical name of the drug matched a list of opioid names (ESM eTable 3). Each drug was assigned one of five categories based on the most commonly prescribed opioids in our data set: codeine, hydromorphone, oxycodone, tramadol, and “other.” If more than one type of opioid was dispensed on the first date of dispensation, the higher potency opioid (i.e., hydromorphone or oxycodone) was selected as the “first” opioid.
Outcomes
Our primary outcome was the percentage (rate) of patients dispensed any opioid within 30 days after surgery per surgical encounter. For comparability with international rates, we also examined rates within seven days after discharge among opioid naïve patients. Other secondary outcomes included morphine milligram equivalents (MME)24 in the first dispensation of any opioid, prescriber preference (the percentage of each opioid type among opioids dispensed or among patients receiving any opioid), percentage of patients receiving multiple opioid dispensations, and duration of opioid prescriptions according to the “dispensed days supply” field in PharmaNet (pharmacists’ estimate of how long the dispensed supply of a medication will last).
Data analysis
We analyzed trends in the four most commonly prescribed types of opioids after each of the five surgery types separately. We grouped all abdominal or all orthopedic surgeries together for analyses of MME: 1) Annual rates: For comparison with international rates, we tabulated unadjusted annual rates. To adjust for trends in age, sex, and opioid dispensation in the year prior to surgery, and to minimize the confounding impact of mean length of stay, we used generalized linear mixed modeling (SAS software’s GLIMMIX procedure; SAS Institute Inc., Cary, NC, USA) to estimate the log of the odds ratio for opioid dispensation in each year relative to the reference year (2016) and its 95% confidence interval (CI), adjusting for clustering of rates within individual hospitals by allowing a random intercept for each hospital. Each odds ratio and its confidence limits were multiplied by the reference odds in 2016 to provide an annual odds, which was converted into an annual percentage with confidence limits; 2) Annual increments: To quantify increases in rates from 2003 to 2016, we measured the average annual increments by entering surgery year as an ordinal variable in the generalized linear mixed model of the log odds ratio, adjusting for autocorrelation of rates over time within individual hospitals, as well as length of stay, age, sex, and prior opioid dispensation; 3) Prescribing preferences: We plotted the annual rates for each type of opioid to identify timing of changes in preference among types, preference for prescribing multiple types, and different prescription durations (measured by days of supply dispensed). In analyses of SQOR Extension data, we also measured preference as a fraction of opioid prescribing, e.g., the conditional probability of prescribing a more potent opioid (hydromorphone or oxycodone) given that any opioid was prescribed; 4) Morphine milligram equivalents: We computed the MME for each patient’s first dispensation using recommended conversion factors for each opioid type from a systematic review24 (codeine, 0.1; hydromorphone, 5.0; oxycodone, 1.5; tramadol, 0.2; others, 0.1 to 39) multiplied by the number of tablets dispensed and mg per tablet. We estimated annual increments in MMEs by multivariable modeling; 5) Individual surgeons: We assessed variation (percentiles and standard deviations) among individual surgeons’ practices in 2016 relative to the number of surgeries of each type that each surgeon performed, excluding those performing fewer than ten such surgeries. We analyzed variation across previous years for high-volume surgeons with relatively low opioid rates in 2016.
The SQOR project used SAS software for extraction, exclusions, analyses, and producing figures. The SQOR Extension project used Golden 6 (Oracle Corporation; Austin, TX, USA) for cohort formation, and RStudio (R Core Team 2016; Boston, MA, USA) for analyses. We used Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to extend SQOR trends, and to compute 95% CI and P values for rates, rate differences, and differences between rate differences.
Results
Table 1 shows the SQOR study cohorts (total N = 236,056) distributed by surgery types and selected years, as well as their characteristics (age, sex, prior dispensation of opioids, and mean length of stay). Only mean length of stay was correlated with both surgery year and opioid rate and therefore a potential confounder.
Annual rates
Table 2 shows selected rates of opioid dispensing by surgery types, opioid type, and prior use of opioids. Between 2003 and 2007, there were steep increases in unadjusted rates after orthopedic surgeries (eFig. 1) that attenuated but did not disappear when length of stay was included in a multivariable model (Fig. 1). Opioid dispensation rates after abdominal surgeries also increased, though more steadily than orthopedic surgeries, and changed little when adjusting for length of stay (Fig. 1). Table 3 shows the slopes of the trends in Fig. 1; the mean annual increases for any opioid ranged from 1% to 2%. There were only negligible differences (–0.3% to 0%) in slope between patients who were or were not dispensed any opioids in the year before surgery.
Percentage of patients dispensed opioids within 30 days after common abdominal and orthopedic surgeries from 2003 to 2016 after adjusting for hospital length of stay and clustering of rates within individual hospitals. Error bars indicate 95% confidence intervals. LA = laparoscopic appendectomy; LC = laparoscopic cholecystectomy; IHR = open inguinal hernia repair; THA = total hip arthroplasty; TKA = total knee arthroplasty
Opioid preferences
Starting in 2007, use of tramadol increased while codeine decreased across all surgery types (Tables 2 and 3, Fig. 2). Rates of hydromorphone and oxycodone dispensation were increasing as early as 2003. After 2007, hydromorphone dispensation continued to increase whereas oxycodone leveled at 22% until 2015, then dropped. The SQOR Extension results showed oxycodone dispensation continued to drop sharply to 5% by 2019, while hydromorphone’s growth slowed but did not fall substantially (ESM eFig. 2). In contrast, oxycodone and hydromorphone were rarely dispensed after abdominal surgeries from 2003 to 2016 (Fig. 3). The percentage of patients receiving more than one type of opioid within seven days rose after orthopedic surgeries but not after abdominal surgeries (Fig. 4).
Percentage of patients dispensed codeine, hydromorphone, oxycodone, and tramadol within 30 days after common abdominal and orthopedic surgeries from 2003 to 2016, after adjusting for hospital of stay and clustering of rates within individual hospitals. Error bars indicate 95% confidence intervals. Abdominal = laparoscopic appendectomy, laparoscopic cholecystectomy, open inguinal or femoral hernia repair; orthopedic = total hip arthroplasty, total knee arthroplasty
Percentage of patients receiving more than one type of opioid dispensations after discharge by surgery type from 2003 to 2016. Error bars indicate 95% confidence intervals. LA = laparoscopic appendectomy; LC = laparoscopic cholecystectomy; IHR = open inguinal hernia repair; THA = total hip arthroplasty; TKA = total knee arthroplasty
Morphine milligram equivalents in the first dispensation of any opioid within 30 days after surgery from 2003 to 2016. LA = laparoscopic appendectomy; LC = laparoscopic cholecystectomy; IHR = open inguinal hernia repair; THA = total hip arthroplasty; TKA = total knee arthroplasty. Error bars indicate 95% confidence intervals
The SQOR Extension study found that, from 2016 to 2019, there was a 60% decrease in dispensations of greater than 11 days supply (ESM eFig. 3). There was a symmetrical increase in dispensations of smaller quantities of less than six days supply. This shift was not observed among lower potency opioids (codeine and tramadol).
Morphine milligram equivalents
Figure 4 shows the mean MME per first dispensation after orthopedic surgeries almost doubled from 2003 to 2013, leveling off in 2014–2016. After abdominal surgeries, MME increased by about half and were nearly equal after LA, LC, and IHR. The contributions to MME trends from trends in number and quantity of tablets are shown in ESM eFigs 4 and 5. Using multivariable regression, allowing for clustering by hospital and practitioner, we found that the mean MME per first dispensation rose by 8 mg⋅yr-1 (95% CI, 7 to 9) (abdominal) and 61 mg⋅yr-1 (95% CI, 58 to 64) (orthopedic). Multiplication of mean MME by the percentage of surgical patients dispensed any opioids postdischarge (Table 2) showed that the MME per abdominal surgery patient doubled from 76 in 2003 to 152 in 2016. The MME per orthopedic patient rose by a factor of 2.5, from 179 in 2003 to 452 in 2016.
Individual surgeons
Table 4 shows the distribution of surgeons by percentiles relative to the percentage of their patients dispensed opioids within 30 days in 2016, excluding surgeons performing fewer than ten of each surgery type. There was greater variation for abdominal surgeries than orthopedic surgeries, but both included noteworthy outliers. Several surgeons (< 6) performed 2,353 abdominal surgeries over 14 years after which only 10% of patients were dispensed opioids, and never more than 18% in any single year. Six orthopedic surgeons who each performed over 100 THAs (n = 1168) with opioid dispensation rates < 75% also had low opioid dispensation rates after TKA (mean = 88%), below the lower quartile. Eight who each performed over 100 TKAs (n = 2,473), with opioid dispensation rates < 85%, also had low opioid dispensation rates after THA (mean = 80%), below the lower quartile.
Discussion
Our overall finding is that rates of opioid dispensation after common abdominal and orthopedic surgeries in BC rose by 1–2% per year since 2003, and the MME of first dispensations also rose steadily as prescribing preferences shifted away from codeine toward higher MME opioids. The combined impact of both trends resulted in the overall MME per abdominal surgery patient doubling from 2003 to 2016, while the MME per orthopedic surgery patient increased by a factor of 2.5.
Opioid dispensation rates after abdominal surgeries in BC in 2016 were comparable to those in Ontario5 and the USA.5,23 Small differences between BC and Ontario rates can be partly explained by different metrics. The mean rates of postdischarge opioid dispensing after abdominal surgeries in BC from 2013 to 2016 among opioid naïve patients were 7% lower than the prescriptions rates in Ontario between 2013 and 2016 as reported by Ladha et al. (ESM eTable 8).5 For prescriptions, they used data from a compulsory Narcotics Monitoring System. Part of the difference in rates might be due to prescriptions not being filled by some patients. This is consistent with a recent study at the North York General Hospital in Ontario that found that, at baseline, the median number of opioid tablets used was only two out of 20 dispensed after LA and LC, and, after an opioid stewardship intervention, was reduced to zero out of ten dispensed.17
Ontario and BC rates were about four times higher than the rate of 16% in Sweden5 among LA and LC patients (ESM eTable 8), and the rate of 5% of patients (63% of whom had LA or LC) who received prescriptions at discharge in a mixture of countries (China, Netherlands, Thailand, Colombia, Mexico, Lebanon, and Brazil).23 Some of the difference is probably due to longer stays in hospital (ESM eTable 8). If 80% of postdischarge tablets are unused by patients in North York, Ontario,17 an extra day in hospital might be sufficient for many Swedish patients to avoid any opioids postdischarge.
A distinctive part of the Canadian context was the regulatory status of tramadol. Tramadol became a Schedule IV drug in the USA in 2014 and in Sweden in 2005, whereas it remained an unscheduled drug in Canada until 2022.5,25–27 Therefore, tramadol was exempt from BC’s Prescription Review Program, which requires duplicate or triplicate prescription forms,28 an inconvenience that could be circumvented by prescribing tramadol.
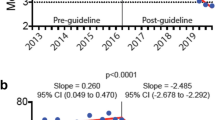
Since the April 2016 declaration of a public health emergency surrounding opioid overuse in BC, we observed a shift from 2016 to 2019 toward fewer tablets dispensed—more dispensations with less than six days supply— of the more potent opioids after orthopedic surgery (ESM eFig. 3). Whether the drop in use of oxycodone (ESM eFig. 2) was influenced by the emergency declaration is unknown. This is consistent with a slight acceleration, from 0.3% to 0.6% per year, in the annual decline in overall percent of the population dispensing of opioids from community pharmacies in BC since 2013, according to the Canadian Institute for Health Information’s National Prescription Drug Utilization Information System.29
Our findings suggest a need for province-wide opioid stewardship during discharge planning and processes, supported by real-time monitoring. The North York study showed feasibility and impact of stewardship.17 Systematic reviews30,31 of prescription monitoring programs in the USA and Canada show that such programs reduce per capita analgesic prescription, with overall fewer MME prescribed in USA states with monitoring programs than without, particularly for high potency opioids. Feedback to individual prescribers comparing their rates of prescribing opioids to opioid-naïve patients was provided to general practitioners in BC in 2020–2021,32 and their prescribing portraits are now online confidentially.33 Prescribing feedback has been shown to have positive impacts in BC.34 Similar feedback could be provided to surgeons through BC’s new Measurement System for Physician Quality Improvement35 by linking surgeons’ billing data to PharmaNet dispensing data.
Limitations
The scope of our study was initially limited to exploring trends in a metric of surgical quality to show feasibility of rapid monitoring. This led to some hypotheses that the study design could address but only indirectly. Our opioid data were limited to dispensations from community pharmacies. That meant we could not separate the prescribers’ decisions from the patients’ decisions. Patients’ tendencies to fill opioid prescriptions could have been changing from 2003 to 2016, especially after the public health emergency declaration, so the trends cannot be entirely attributed to physicians’ prescribing preferences. We also lacked data on opioid use in hospital. Our extract of PharmaNet data could not be linked by physician number to surgeon billing data, but ESM eFigs 6 and 7 show that 90% of first dispensations were on the date of discharge, implying an in-hospital prescriber. We did not exclude patients who were chronic users of opioids in the year before surgery but stratified on such prior use and found that slopes were similar (ESM eFig. 8). Further data on patients’ comorbidities and surgeon and prescriber characteristics would be needed for fair comparisons among individual surgeons. Premature termination of the SQOR Extension study because of the COVID-19 pandemic prevented computation of MME and multivariable analyses of cohorts in the years 2017 through 2019, and meant the only available metric of prescription size after 2016 was “days supply.”
Conclusions
The data from our study show a rise by 1% to 2% per year from 2003 to 2016 of postdischarge opioid dispensing after common abdominal and orthopedic surgeries in BC that was not an artifact of shorter lengths of stay in hospital. It was accompanied by steadily increasing MMEs in first dispensations of opioids. Together, these trends doubled the MME per patient from 2003 to 2016. Laparoscopic appendectomy and LC patients in BC who had not used an opioid in the previous year were four times more likely to receive postdischarge opioids than patients undergoing identical procedures in Sweden were. The rise in tramadol dispensation since 2005 might be attributable partly to Health Canada’s delay in classifying it as a controlled substance until 2022. Our findings support the feasibility of rapid monitoring of postdischarge opioid rates, linked to surgeon billings, and the need for it to be part of an opioid stewardship strategy.
References
Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. BMJ 2018; https://doi.org/10.1136/bmj.j5790.
Howard R, Waljee J, Brummett C, Englesbe M, Lee J. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg 2018; 153: 285–7.
Overton HN, Hanna MN, Bruhn WE, et al. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg 2018; 227: 411–8.
International Narcotics Control Board. Report of the International Narcotics Control Board for 2019. Available from URL: https://www.incb.org/incb/en/publications/annual-reports/annual-report-2019.html (accessed April 2022).
Ladha KS, Neuman MD, Broms G, et al. Opioid prescribing after surgery in the United States, Canada, and Sweden. JAMA Netw Open 2019; https://doi.org/10.1001/jamanetworkopen.2019.10734.
Bai J-W, Bao J, Bhatia A, Chan VWS. A perioperative approach to the opioid crisis. CMAJ 2018; 190: E1151–2.
Waljee JF, Li L, Brummett CM, Englesbe MJ. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg 2017; 265: 728–30.
Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US Adults. JAMA Surg 2017; https://doi.org/10.1001/jamasurg.2017.0504.
Howard R, Gunaseelan V, Brummett C, Waljee J, Englesbe M, Telem D. New persistent opioid use after inguinal hernia repair. Ann Surg 2020; https://doi.org/10.1097/sla.0000000000004560.
Feinberg AE, Chesney TR, Srikandarajah S, Acuna SA, McLeod RS, Best Practice in Surgery Group. Opioid use after discharge in postoperative patients: a systematic review. Ann Surg 2018; 267: 1056–62.
Clarke H, Soneji N, Ko DT, Yun L, Wijeysundera DN. Rates and risk factors for prolonged opioid use after major surgery: population based cohort study. BMJ 2014; https://doi.org/10.1136/bmj.g1251.
Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med 2012; 172: 425–30.
Canadian Institute for Health Information. Hospital stays in Canada 2022. Available from URL: https://www.cihi.ca/en/hospital-stays-in-canada (accessed April 2022).
Goesling J, Moser SE, Zaidi B, et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016; 157: 1259–65.
Bicket MC, Long JJ, Pronovost PJ, Alexander GC, Wu CL. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg 2017; 152: 1066–71.
Hill MV, McMahon ML, Stucke RS, Barth RJ Jr. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg 2017; 265: 709–14.
Feinberg AE, Acuna SA, Smith D, et al. Optimizing opioid prescriptions after laparoscopic appendectomy and cholecystectomy. Can J Surg 2021; 64: E69–75.
Canadian Patient Safety Institute. Enhanced Recovery CanadaTM 2022. Available from URL: https://www.patientsafetyinstitute.ca/en/toolsResources/Enhanced-Recovery-after-Surgery/Pages/default.aspx (accessed April 2020).
Population Data BC. Medical services plan data set 2022. Available from URL: https://www.popdata.bc.ca/data/health/msp (accessed April 2022).
Population Data BC. Discharge abstracts database (hospital separations) data set. Available from URL: https://www.popdata.bc.ca/data/health/dad (accessed April 2022).
Population Data BC. PharmaNet data set 2022. Available from URL: https://www.popdata.bc.ca/data/health/pharmanet (accessed April 2022).
Population Data BC. Central demographics file (MSP registration and premium billings, client roster and census geodata)/Consolidation file (MSP registration and premium billing) data set. Available from URL: https://www.popdata.bc.ca/data/demographic/consolidation_file (accessed April 2022).
Kaafarani HMA, Han K, El Moheb M, et al. Opioids after surgery in the United States versus the rest of the world: the international patterns of opioid prescribing (iPOP) multicenter study. Ann Surg 2020; 272: 879–86.
Nielsen S, Degenhardt L, Hoban B, Gisev N. A synthesis of oral morphine equivalents (OME) for opioid utilisation studies. Pharmacoepidemiol Drug Saf 2016; 25: 733–7.
Government of Canada. Canada Gazette, Part 1, Volume 153, Number 16: Regulations amending the narcotic control regulations (Tramadol) 2022. Available from URL: http://www.gazette.gc.ca/rp-pr/p1/2019/2019-04-20/html/reg2-eng.html (accessed April 2022).
Government of Canada. Regulations amending the narcotic control regulations (Tramadol): SOR/2021-31, 2022. Available from URL: https://canadagazette.gc.ca/rp-pr/p2/2021/2021-03-31/html/sor-dors43-eng.html (accessed April 2022).
Canadian Pharmacists Association. CPhA policy 2022. Available from URL: https://www.pharmacists.ca/advocacy/policy-position-statements/tramadol-1.5134762 (accessed April 2022).
College of Pharmacists of British Columbia. Controlled prescription program. Available from URL: https://www.bcpharmacists.org/cpp (accessed April 2022).
Canadian Institute for Health Information. Opioid prescribing in canada: how are practices changing? 2019. Available from URL: https://www.cihi.ca/sites/default/files/document/opioid-prescribing-canada-trends-en-web.pdf (accessed April 2022).
Wilson MN, Hayden JA, Rhodes E, Robinson A, Asbridge M. Effectiveness of prescription monitoring programs in reducing opioid prescribing, dispensing, and use outcomes: a systematic review. J Pain 2019; 20: 1383–93.
Moride Y, Lemieux-Uresandi D, Castillon G, et al. A systematic review of interventions and programs targeting appropriate prescribing of opioids. Pain Physician 2019; 22: 229–40.
Klimas J, Hamilton MA, Carney G, et al. Individualized prescribing portraits to reduce inappropriate initiation of opioid analgesics to opioid naïve patients in primary care: protocol for a randomized controlled trial. Contemp Clin Trials 2021; https://doi.org/10.1016/j.cct.2021.106462.
Therapeutics Initiative. Portrait: Sparing opioid prescription to opioid naive patients. Available from URL: https://www.ti.ubc.ca/2021/06/02/sparing-opioid-prescription-to-opioid-naive-patients/ (accessed June 5, 2022).
Dormuth CR, Carney G, Taylor S, Bassett K, Maclure M. A randomized trial assessing the impact of a personal printed feedback portrait on statin prescribing in primary care. J Contin Educ Health Prof 2012; 32: 153–62.
Doctors of BC. MSPQI - creating quality improvement measurements that empower physicians 2019. Available from URL: www.doctorsofbc.ca/news/mspqi-creating-quality-improvement-measurements-empower-physicians (accessed April 2022).
Author contributions
Parker Nann contributed to drafting the manuscript and to analysis and interpretation of the data. Noushin Nabavi contributed to analysis and interpretation of the data. Kimia Ziafat contributed to drafting the manuscript and interpretation of the data. Roanne Preston contributed to study conception and design, acquisition of grant funding and data, study management, interpretation of data, and revisions to the manuscript. Anthony Chau contributed to conception and design, analysis and interpretation of the data, and manuscript writing. Michael Krausz and Stephan Schwarz contributed to the convening of the author group, funding, conceptualization, and design, interpretation of data, and manuscript writing. Malcolm Maclure contributed to all aspects, including study conception and design, acquisition of grant funding and data, study management, analysis and interpretation of data, and writing and revising the manuscript.
Disclosures
Dr. Stephan K. W. Schwarz is the Editor-in-Chief of the Canadian Journal of Anesthesia/Journal canadien d’anesthésie; he had no involvement in the handling of this manuscript. All other authors declare no other associations.
Disclaimers
All inferences, opinions, and conclusions drawn in this publication are those of the author(s) and do not reflect the opinions or policies of the Data Steward(s), or any institution or funder.
Funding statement
This research was funded by a grant from the Partnerships for Health System Improvement of the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research grant (funding number: 0407004979) and by the Ministry of Health of British Columbia. Dr. Maclure is supported as the BC Chair in Patient Safety at The University of British Columbia (Vancouver, BC, Canada) by an endowment from the BC Ministry of Health (Victoria, BC, Canada) and was partially supported as a Scholar-in-Residence in the BC Ministry of Health from 2018 to 2020. Dr. Krausz holds the UBC-Providence Leadership Chair for Addiction Research (Vancouver, BC, Canada). Dr. Schwarz holds the Dr. Jean Templeton Hugill Chair in Anesthesia, supported by the Dr. Jean Templeton Hugill Endowment for Anesthesia Memorial Fund at The University of British Columbia (Vancouver, BC, Canada).
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie.
Data availability
Access to data provided by the Data Steward(s) is subject to approval, but can be requested for research projects through the Data Steward(s) or their designated service providers.
Author information
Authors and Affiliations
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nann, P., Nabavi, N., Ziafat, K. et al. Trends in opioid dispensing after common abdominal and orthopedic surgery procedures in British Columbia: a retrospective cohort analysis. Can J Anesth/J Can Anesth 69, 986–996 (2022). https://doi.org/10.1007/s12630-022-02272-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-022-02272-7