Abstract
Purpose
There is some evidence for the use of intrathecal morphine as a means to provide prolonged analgesia in selective cardiac surgical patients; however, the hemodynamic effects of intrathecal morphine are not well defined. This study was designed to study the effect of intrathecal morphine on hemodynamic parameters in cardiac surgery patients.
Methods
In a prospective, double-blind study, 100 adult cardiac surgical patients were randomized to receive either intrathecal 40 mg of 0.5% hyperbaric bupivacaine alone (intrathecal bupivacaine [ITB] group, n = 50) or intrathecal 250 µg of morphine added to 40 mg of 0.5% bupivacaine (intrathecal bupivacaine and morphine [ITBM] group, n = 50). Hemodynamic data, pain scores, rescue analgesic use, spirometry, and vasopressor use were recorded every four hours after surgery for 48 hr. The primary outcome was the incidence of vasoplegia in each group, which was defined as a cardiac index > 2.2 L·min-1·m-2 with the requirement of vasopressors to maintain the mean arterial pressure > 60 mmHg with the hemodynamic episode lasting > four hours.
Results
Eighty-seven patients were analyzed (ITB group, n = 42, and ITBM group, n =45). The incidence of vasoplegia was higher in the ITBM group than in the ITB group [14 (31%) vs 5 (12%), respectively; relative risk, 2.6; 95% confidence interval [CI], 1.0 to 6.6; P = 0.04]. The mean (standard deviation [SD]) duration of vasoplegia was significantly longer in the ITBM group than in the ITB group [8.9 (3.0) hr vs 4.3 (0.4) hr, respectively; difference in means, 4.6; 95% CI, 3.7 to 5.5; P < 0.001].
Conclusion
Intrathecal morphine added to bupivacaine for high spinal anesthesia increases the incidence and duration of vasoplegia in cardiac surgery patients.
Trial registration
www.clinicaltrials.gov (NCT02825056); registered 19 June 2016.
Résumé
Objectif
Certaines données probantes appuient l’utilisation de morphine intrathécale pour une analgésie prolongée chez certains patients de chirurgie cardiaque; cependant, les effets hémodynamiques de la morphine intrathécale ne sont pas bien définis. Cette étude a été conçue pour évaluer l’effet de la morphine intrathécale sur les paramètres hémodynamiques de patients de chirurgie cardiaque.
Méthode
Dans une étude prospective et à double insu, 100 patients adultes de chirurgie cardiaque ont été randomisés à recevoir 40 mg de bupivacaïne hyperbare 0,5 % intrathécale (groupe bupivacaïne intrathécale [BIT], n = 50) ou 250 μg de morphine intrathécale ajoutés à 40 mg de bupivacaïne 0,5 % (groupe bupivacaïne et morphine intrathécales [BMIT], n = 50). Les données hémodynamiques, les scores de douleur, l’utilisation d’analgésiques, la spirométrie et l’utilisation de vasopresseurs ont été enregistrés toutes les quatre heures après la chirurgie pendant 48 heures. Le critère d’évaluation principal était l’incidence de vasoplégie dans chaque groupe, définie comme un index cardiaque > 2,2 L·min-1·m2 nécessitant des vasopresseurs pour maintenir la tension artérielle moyenne > 60 mmHg avec une durée de plus de quatre heures.
Résultats
Quatre-vingt-sept patients ont été analysés (groupe BIT, n = 42, et groupe BMIT, n = 45). L’incidence de vasoplégie était plus élevée dans le groupe BMIT que dans le groupe BIT [14 (31%) vs 5 (12 %), respectivement; risque relatif, 2,6; intervalle de confiance [IC] 95 %, 1,0 à 6,6; P = 0,04]. La durée moyenne (écart type [ÉT]) de la vasoplégie était significativement plus longue dans le groupe BMIT que dans le groupe BIT [8,9 (3,0) h vs 4,3 (0,4) h, respectivement; différence de moyennes, 4,6; IC 95 %, 3,7 à 5,5; P < 0,001].
Conclusion
L’ajout de morphine intrathécale à la bupivacaïne pour une anesthésie rachidienne haute augmente l’incidence et la durée de la vasoplégie chez les patients de chirurgie cardiaque.
Enregistrement de l’étude
www.clinicaltrials.gov; (NCT02825056); enregistrée le 19 juin 2016.
Similar content being viewed by others
Cardiac surgery and cardiopulmonary bypass (CPB) are associated with adrenergic stress, resulting in a systemic inflammatory tissue response that may contribute to the development of several adverse postoperative outcomes including myocardial dysfunction, respiratory failure, renal and neurologic dysfunction, bleeding disorders, altered liver function, and multiple organ failure.1,2,3 The traditional approach is to use general anesthesia (GA) supplemented by potent opioids, but it does not completely block this stress response.4,5 Use of high-dose opioid techniques result in prolonged ventilation, postoperative pulmonary complications (PPCs) and prolonged intensive care unit (ICU) stays, and are no longer standard of care for cardiac surgery.6,7
Central neuraxial blocks combined with GA has been shown to attenuate the adrenergic stress response more effectively.8,9,10,11 In this era of enhanced recovery after surgery, regional anesthesia is being increasingly used in cardiac surgery for pain control and to decrease the stress response.10,11,12,13 Although there is a theoretical risk of spinal hematoma in patients who will get heparinized during surgery, the evidence does not support this increased risk when the block is placed at least one hour before anticoagulation.10,14,15 Intrathecal morphine could be an attractive way of providing prolonged analgesia in patients undergoing cardiac surgery and is associated with less postoperative opioid use, decreased time to extubation, and a decrease in PPCs.13 Although neuraxial anesthesia with intrathecal morphine has been studied in cardiac surgery, there is a dearth of literature regarding the hemodynamic effects of intrathecal morphine in combination with local anesthetics in cardiac surgery patients.9,10,11,12,13,16,17
Vasoplegia after cardiac surgery is characterized by significant hypotension, high or normal cardiac index, low systemic vascular resistance, and increased requirements for fluids and vasopressors during or after CPB. With an incidence of 5–25%, it is difficult to treat and is associated with increased morbidity and mortality.18,19 In this study, we evaluated if intrathecal morphine in combination with local anesthetics has vasoplegic effects in patients undergoing cardiac surgery.
In our cardiac centre at the Post Graduate Institute of Medical Education and Research (PGIMER) in Chandigarh India and at St. Boniface Hospital Winnipeg, Canada, we frequenty use high spinal anesthesia, with spinal blockade up to the T1 level, combined with light GA, for suitable patients undergoing cardiac surgery. Invasive pressure monitoring of cardiac surgical patients and the timely use of bolus or continuous infusion of vasoactive agents like phenylephrine, norepinephrine, or epinephrine as per the hemodynamic requirements of underlying cardiac lesions enable successful use of such a technique.8,9,10,11,12,16 The primary objective of this study was to compare the incidence of vasoplegia in two groups of patients, with one group receiving intrathecal local anesthetic and the other group receiving intrathecal morphine added to the local anesthetic. Secondary objectives included the time to extubation, postoperative rescue analgesic requirement, spirometry performance, and occurrence of any adverse event associated with intrathecal morphine (e.g., pruritus, nausea/vomiting, respiratory depression, and neuro-deficit) in the two groups.
Methods
This prospective, randomized, double-blind clinical study was conducted at PGIMER, Chandigarh, India, after Institutional Ethical Review Board approval (NK-2676-DM-397) and written informed consent of all participants. This trial was registered prior to patient enrolment at clinicaltrials.gov (NCT02825056).
Patients aged between 18 and 60 yr, undergoing elective cardiac surgery were included in the study. Exclusion criteria were local site infection or spinal deformity, coagulopathy (platelet count < 80,000/dL, international normalized ratio > 1.5), redo/emergency cardiac surgeries, obesity (body mass index > 30 kg·m−2), anticipated total surgery time > six hours, chronic obstructive pulmonary disease, asthma, opioid drug abuse or opioid tolerance, and known or anticipated difficult airway. Patients with a failed spinal block (hemodynamic response to skin incision and sternotomy) and poor transthoracic echocardiography (TTE) window in the postoperative period were excluded from the analysis.
All patients received their usual cardiovascular medications except angiotensin-converting enzyme (ACE) inhibitors, digoxin, and diuretics on the morning of surgery. In the operating room, after securing peripheral intravenous and radial artery line, participants received either 40 mg of 0.5% hyperbaric bupivacaine with 0.25 mL normal saline [intrathecal bupivacaine (ITB) group] or 40 mg of 0.5% hyperbaric bupivacaine with 250 µg of morphine in 0.25 mL of normal saline [intrathecal bupivacaine and morphine (ITBM) group] intrathecally using a 26G Quincke needle at L3–L4 level in lateral or sitting position and then turned back to supine position with 10° Trendelenburg tilt to achieve high thoracic block. Subsequently, GA was induced, and the central line was placed through the right internal jugular vein. Phenylephrine and norepinephrine were used in stenotic lesions and coronary artery disease with normal left ventricular (LV) function. Epinephrine was used in patients with regurgitant lesions, dilated LV, or low LV function. Low dose milrinone (0.2–0.25 µg·kg-1·min-1) was added in patients with low LV function. The overall selection of drugs to manage hemodynamics was left to the discretion of attending anesthesiologists in the operating room and treating physicians in the ICU, both of whom were blinded to the drugs used in the spinal.
General anesthesia was induced in all patients with ketamine 20–30 mg plus lidocaine 2 mg·kg-1 and propofol titrated until the eyelash reflex was lost. Vecuronium bromide 0.1 mg·kg-1 was used as a muscle relaxant to facilitate tracheal intubation. Subsequent anesthesia was maintained in all patients with isoflurane to maintain bispectral index values at 40–60. Patients were mechanically ventilated with 50% oxygen-air mixture, and minute ventilation was adjusted to maintain normocapnia.
Pre-CPB transesophageal echocardiography measurement of left ventricle outflow tract (LVOT) diameter in mid-esophageal long-axis view was recorded. The average of three readings was used to calculate the LVOT area and the cardiac index throughout the study. A normothermic bypass was used in all patients, and hematocrit was maintained ≥ 24% on bypass and ≥ 30% post-surgery.
In the ICU, iv paracetamol 1 g every six hours was given to all patients for 48 hr postoperatively. Fentanyl (10–20 µg iv) and morphine (2–3 mg iv) were used as rescue analgesia. The transthoracic echocardiography apical five-chamber view was used to calculate the LVOT velocity time integral (VTI) in the ICU. The following parameters were recorded every four hours until 48 hr after completion of CPB: heart rate (HR), mean arterial pressure (MAP), cardiac index, dose of vasoactive drugs, ten-point visual analogue scale (VAS) score for pain,20 and the dose of rescue analgesics administered. Since all patients were monitored continuously by electrocardiogram and invasive arterial pressure during the study period, we recorded the stabilized values of HR and MAP around proposed recording time points in resting patients. Vasoplegia was defined as a cardiac index > 2.2 L·min-1·m-2 with the requirement of vasopressors to maintain the MAP > 60 mmHg, with the hemodynamic episode lasting for more than four hours.21 The time to extubation, postoperative spirometry at one-hour post-extubation and 24 and 48 hr post-CPB, and the occurrence of complications (pruritus, nausea/vomiting, respiratory depression, and neuro-deficit) were also noted. Further, vasoactive inotropic score (VIS) was calculated at specific time intervals using the following formula: VIS = dopamine dose (µg·kg-1·min-1) + dobutamine dose (µg·kg-1·min-1) + 100 × epinephrine dose (µg·kg-1·min-1) + 10 × milrinone (µg·kg-1·min-1) + 10,000 × vasopressin dose (U·kg-1·min-1) + 100 × norepinephrine dose (µg·kg-1·min-1).22
Statistical analysis
The sample size was calculated based on a pilot feasibility study with ten patients in each group. After analyzing the data of this pilot feasibility study, we found vasoplegia incidence to be 10% in the ITB group and 40% in the ITBM group. Assuming an alpha error of 5%, the beta error of 15% and the power of the study to be 85%, the total sample size for the study was 72 patients, with 36 patients to be included in each group. After the sample size calculation, it was decided to include 100 patients within the time frame of the project.
Randomization was done using a sealed envelope method, and the randomization sequence was generated using www.randomizer.org. On the day of surgery, one of the envelopes was picked up by an anesthesiologist not involved in the study, who prepared the drug aseptically and handed over the syringe to the anesthesiologist who administered the intrathecal block. After entering patient information, the envelope was sealed by an anesthesiologist not involved in the study and handed over to the chief theatre technician who maintained them until the end of patient enrolment. The data were analyzed group-wise before the assignment was known to the investigator.
Continuous variables were expressed as mean (standard deviation [SD]) or median [interquartile range], and dichotomous data were expressed as numbers and percentages. The normality of distribution of the continuous data were tested with the Kolmogorov–Smirnov one-sample test. We employed Student’s t test for normally distributed continuous data. Data that did not have a normal distribution, as well as ordinal data, were analyzed with the Mann–Whitney U test with Bonferroni correction. For categorical data, Pearson’s Chi square test was used. When the expected count was less than five, Fisher’s exact test was used. The hemodynamic data recorded at various time intervals were analyzed using repeated measure analysis of variance. A P value of < 0.05 was considered significant for all statistical tests. Statistical analysis was performed using SPSS (IBM Statistical Package for the Social Sciences 21, Chicago, IL, USA) for Windows.
Results
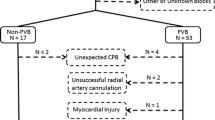
One hundred eligible consenting patients were randomized in the study, with 50 patients in each group, from July 2016 to February 2017. Both groups were comparable in patient demographics, comorbidities, investigations, preoperative medication, type of surgery, and CPB parameters (Table 1). The final analysis was done in 42 patients in the ITB group and 45 patients in the ITBM group (as shown in the CONSORT diagram in Fig. 1). None of these 87 patients analyzed showed any hemodynamic response to skin incision and sternotomy, confirming the efficacy of block in all the patients.
The primary outcome of the study, the incidence of vasoplegia, was higher in the ITBM group than in the ITB group (14 [31%] vs 5 [12%], respectively; relative risk, 2.6; 95% CI, 1.0 to 6.6; P = 0.04). The mean (SD) duration of vasoplegia was significantly longer in the ITBM group than in the ITB group [8.9 (3.0) hr vs 4.3 (0.4) hr, respectively; differences of mean, 4.6; 95% CI, 3.7 to 5.5; P < 0.001] (Table 2). Mean (SD) time to extubation was not significantly different between the two groups [4.1 (2.9) in ITBM vs 4.9 (2.4) in ITB; differences of mean, –0.8; 95% CI, -1.9 to 0.4; P = 0.19]. The peak inspiratory flow achieved on incentive spirometry at one-hour post-extubation and at 24 and 48 hr after surgery were better in the ITBM group, and the requirement of rescue analgesics was also lower in the ITBM group (Table 2). The VAS score was higher in the ITB group than in the ITBM group throughout the 48-hr study period (Table 3). The total doses of vasopressors and inotropes represented by the total VIS score were not significantly different between the two groups (Table 4). There was no difference in the incidence of nausea/vomiting and pruritus between the groups, and none of the patients in the study had respiratory depression or postoperative neurologic deficit.
The HR, MAP, and cardiac index were comparable between the groups at all time intervals throughout the 48-hr study period (Fig. 2). At eight hours post-ICU admission, the mean (SD) MAP was lowest in the ITBM group, but was not statistically different to the mean MAP values in the ITB group; [73.8 (15.5) vs 78.9 (11.6), respectively; differences of mean, -5.0; 95% CI, -11.0 to 0.8; P = 0.09].
Discussion
This single-centre study showed a significantly higher incidence and prolonged duration of vasoplegia in patients receiving intrathecal morphine with bupivacaine for high spinal block compared with the control group. It has been observed that morphine and other opioids have local anesthetic like action in internally perfused squid giant axons and in mammalian peripheral and central nerve fibres.23,24,25 Epidural morphine inhibits both cardiac and renal sympathetic nerve activity and simultaneously reduces blood pressure and HR in anesthetized cats, and these effects can be reversed by naloxone.26 Clinical studies also support the sympatholytic action of intrathecal opioids. In a study comparing intrathecal opioids and epidural bupivacaine, similar vasodilation was observed in both the groups, when observed using calf-toe temperature gradient and pulse wave plethysmography.27 The sympatholytic activity has been suggested to occur following epidural morphine administration also. In patients undergoing abdominal aortic surgeries, plasma norepinephrine levels were lower in the group receiving epidural morphine than in a group receiving epidural saline and parenteral analgesia.28 One explanation for this may be better pain relief by the central neuraxial morphine. Even in cardiac surgery patients, there was a trend towards lower plasma norepinephrine and epinephrine levels after a large dose of morphine was injected into the intrathecal space.17
Vasoplegia after cardiac surgery is multifactorial. Various risk factors for vasoplegia are patients with renal failure, previous cardiac surgery, combined coronary artery bypass, and valve surgery, higher use of red blood cells, longer aortic cross clamp, and CPB duration.29 Some studies have shown a higher incidence of vasoplegia with preoperative use of ACE inhibitors, beta-blockers, high comorbidities, low LV function, and duration of CPB.18,30 These factors were equally distributed in our study in both the groups and were unlikely to add bias to our findings.
The definition of vasoplegia described by Colson et al.21 did not mention the duration or the use of vasopressors. Nevertheless, we included these criteria in our definition of vasoplegia for the following reasons: 1) use of vasopressors to treat vasodilation-related hypotension is the sine-qua-non of good hemodynamic management, 2) the effect of local anesthetic bupivacaine towards vasoplegia needed to be ruled out, and 3) transient episodes of vasodilation due to other reasons may occur after cardiac surgery that can be treated with boluses of vasopressors. Despite higher incidence and longer duration of vasoplegia in patients who received intrathecal morphine, the amount of vasopressors in terms of the VIS score needed to treat vasoplegia was similar in the two groups. The reasons for this could be: 1) different weightage to different vasopressors when calculating the VIS score, and 2) that the choice of vasopressor and the time to titrate down the vasopressor were at the discretion of the treating physician. Another potential reason could be that the only lower limit of the MAP at 60 mmHg was set without any higher limit for treatment.
Not surprisingly, we found that intrathecal morphine decreased the postoperative pain scores as well as the use of iv opioids. Intrathecal morphine provides better postoperative analgesia.31 Whether this improvement in pain scores results in improved outcomes after cardiac surgery is not known.8,10,12,13,32
Another major concern with the use of intrathecal morphine is respiratory depression. In our study, intrathecal morphine did not increase the time to extubation, which was comparable in both groups, and no patient required reintubation. This may be because of the low dose of morphine (< 0.5 mg) used. These findings are in agreement with two earlier meta-analyses of cardiac surgery patients receiving intrathecal morphine.10,31
The results of a study by Ellenberger et al.13 suggested that improving postoperative analgesia with a small dose of spinal morphine while minimizing systemic opioid administration may reduce the incidence of PPCs in patients undergoing elective cardiac surgery. We did not measure the incidence of PPCs and thus cannot comment on this. We only recorded postoperative spirometry performance, which was better in the ITBM group at all time points, similar to a previous study which reported excellent analgesia with intrathecal morphine resulting in improved postoperative pulmonary function tests.33
The primary concern with high spinal for cardiac surgery is its safety because these patients are fully heparinized, and there is a potential risk of spinal hematoma. To date, there is a single case of spinal hematoma following the full heparinization associated with CPB in a patient who received an epidural.34 The most recent guidelines from the American Society of Regional Anesthesia and Pain Medicine recommend certain precautions to minimize the risk but do not make CPB a contraindication to the use of neuraxial techniques.15 None of the patients in our study had neurologic sequel post-spinal anesthesia; however, our study was not powered to investigate the safety of spinals and we cannot recommend routine use of these in cardiac surgical patients.
The findings of our study are of relevance to fast-tracking and management of cardiac surgical patients receiving intrathecal morphine in postoperative cardiac ICU where they get fast-track extubation (within six hours) or extubation even in the operating room. The increased incidence and duration of vasoplegia in uncomplicated patients who received intrathecal morphine will require an important paradigm shift in their postoperative ICU management similar to that in the management of post central neuraxial block hypotension for which vasopressors are considered the first line of management in addition to titrated fluid supplementation and not only fluid boluses.35,36 The results of this study show that, despite increased incidence and duration of vasoplegia, suitably chosen cardiac surgical patients will benefit from intrathecal morphine.
Our study also has some important limitations. First, it is a single-centre experience, and the results cannot necessarily be generalized. Second, the treatment of vasoplegia was left to the clinical judgement of the treating physician without a higher MAP target. Thus, it is possible that unmeasured confounding factors, such as choice of vasopressor use, could have differed between the groups and therefore affected the results. Third, our study can also be criticized for excluding chronic obstructive pulmonary disease and obese patients; however, this was because we used TTE for LVOT VTI measurement during the immediate postoperative period. These patients would have been excluded in high numbers because of their poor TTE acoustic window. Fourth, we did not take the length of ICU and hospital stay and cost-effectiveness of intrathecal morphine into account. A more comprehensive, multi-institutional study involving a larger group of patients may be useful to establish the cost-benefit ratio of intrathecal morphine.
In conclusion, this prospective randomized study showed that intrathecal morphine added to bupivacaine for high spinal block increased the incidence and duration of vasoplegia in cardiac surgery patients without affecting the quantity of cumulative vasopressor use and the duration of ventilation.
References
Cremer J, Martin M, Redl H, et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996; 61: 1714-20.
Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003; 75: S715-20.
Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg 2002; 21: 232-44.
Haessler R, Schwender D, Leppmeier U, Klasing S, Rindfleisch F, Peter K. Anaesthesia for coronary artery bypass grafting: opioid-analgesia combined with either flunitrazepam, propofol or isoflurane. Acta Anaesthesiol Scand 1993; 37: 532-40.
Philbin DM, Rosow CE, Schneider RC, Koski G, D’Ambra MN. Fentanyl and sufentanil anesthesia revisited: how much is enough? Anaesthesiology 1990; 73: 5-11.
Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother 2007; 41: 400-6.
Meade MO, Guyatt G, Butler R, et al. Trials comparing early vs late extubation following cardiovascular surgery. Chest 2001; 120(6 Suppl): 445S-53S.
Mertin S, Sawatzky JA, Diehl-Jones WL, Lee TW. Total spinal anesthesia for cardiac surgery: does it make a difference in the patient outcomes? Dynamics 2009; 20: 18-24.
Lee TW, Grocott HP, Schwinn D, Jacobsohn E. High spinal anesthesia for cardiac surgery: effects on beta-adrenergic receptor function, stress response, and hemodynamics. Anesthesiology 2003; 98: 499-510.
Liu SS, Block BM, Wu CL. Effects of perioperative central neuraxial analgesia on outcome after coronary artery bypass surgery: a meta-analysis. Anesthesiology 2004; 101: 153-61.
Lee TW, Kowalski S, Falk K, Maguire D, Freed DH, HayGlass KT. High spinal anesthesia enhances anti-inflammatory responses in patients undergoing coronary artery bypass graft surgery and aortic valve replacement: randomized pilot study. PLoS One 2016; DOI: https://doi.org/10.1371/journal.pone.0149942.
Hanada S, Kurosawa A, Randall B, Van Der Horst T, Ueda K. Impact of high spinal anesthesia technique on fast-track strategy in cardiac surgery: retrospective study. Reg Anesth Pain Med 2020; 45: 22-6.
Ellenberger C, Sologashvili T, Bhaskaran K, Licker M. Impact of intrathecal morphine analgesia on the incidence of pulmonary complications after cardiac surgery: a single center propensity-matched cohort study. BMC Anesthesiol 2017; 17: 109.
Ho AM, Chung DC, Joynt GM. Neuraxial blockade and hematoma in cardiac surgery: estimating the risk of a rare adverse event that has not (yet) occurred. Chest 2000; 117: 551-5.
Horlocker TT, Vandermeuelen E, Kopp SL, Gogarten W, Leffert LR, Benzon HT. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine evidence-based guidelines (Fourth Edition). Reg Anesth Pain Med 2018; 43: 263-309.
Kowalewski RJ, MacAdams CL, Eagle CJ, Archer DP, Bhardwaj B. Anaesthesia for coronary artery bypass surgery supplemented with subarachnoid bupivacaine and morphine: a report of 18 cases. Can J Anaesth 1994; 41: 1189-95.
Chaney MA, Smith KR, Barclay JC, Slogoff S. Large-dose intrathecal morphine for coronary artery bypass grafting. Anesth Analg 1996; 83: 215-22.
Levin MA, Lin HM, Castillo JG, Adams DH, Reich DL, Fischer GW. Early on–cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation 2009; 120: 1664-71.
Carrel T, Englberger L, Mohacsi P, Neidhart P, Schmidli J. Low systemic vascular resistance after cardiopulmonary bypass: incidence, etiology, and clinical importance. J Card Surg 2000; 15: 347-53.
Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int 1985; 5: 145-8.
Colson PH, Bernard C, Struck J, Morgenthaler NG, Albat B, Guillon G. Post cardiac surgery vasoplegia is associated with high preoperative copeptin plasma concentration. Crit Care 2011; DOI: https://doi.org/10.1186/cc10516.
Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010; 11: 234-8.
Frazier DT, Murayama K, Abbott NJ, Narahashi T. Effect of morphine on internally perfused squid giant axons. Proc Soc Exp Biol Med 1972; 139: 434-8.
Kosterlitz HW, Wallis DI. The action of morphine-like drugs on impulse transmission in mammalian nerve fibres. Br J Pharmcol Chemother 1964; 22: 499-510.
Gissen AJ, Gugino LD, Datta S, Miller J, Covino BG. Effect of fentanyl and sufentanil on peripheral mammalian nerves. Anesth Analg 1987; 66: 1272-6.
Mori T, Nishikawa K, Terai T, Yukioka H, Asada A. The effects of epidural morphine on cardiac and renal sympathetic nerve activity in α-chloralose-anesthetized cats. Anesthesiology 1998; 88: 1558-65.
Goodarzi M, Narasimhan RR. The effect of large-dose intrathecal opioids on the autonomic nervous system. Anesth Analg 2001; 93: 456-9.
Breslow MJ, Jordan DA, Christopherson R, et al. Epidural morphine decreases postoperative hypertension by attenuating sympathetic nervous system hyperactivity. JAMA 1989; 261: 3577-81.
Dayan V, Cal R, Giangrossi F. Risk factors for vasoplegia after cardiac surgery: a meta-analysis. Interact Cardiovasc Thorac Surg 2019; 28: 838-44.
Weis F, Kilger E, Beiras-Fernandez A, et al. Association between vasopressor dependence and early outcome in patients after cardiac surgery. Anaesthesia 2006; 61: 938-42.
Meylan N, Elia N, Lysakowski C, Tramer MR. Benefit and risk of intrathecal morphine without local anaesthetic in patients undergoing major surgery: meta-analysis of randomized trials. Br J Anaesth 2009; 102: 156-7.
Mangano DT, Siliciano D, Hollenberg M, et al. Postoperative myocardial ischemia. Therapeutic trials using intensive analgesia following surgery. The Study of Perioperative Ischemia (SPI) Research Group. Anesthesiology 1992; 76: 342-53.
Bowler I, Djaiani G, Abel R, Pugh S, Dunne J, Hall J. A combination of intrathecal morphine and remifentanil anesthesia for fast-track cardiac anesthesia and surgery. J Cardiothorac Vasc Anesth 2002; 16: 709-14.
Rosen DA, Hawkinberry DW 2nd, Rosen KR, Gustafson RA, Hogg JP, Broadman LM. An epidural hematoma in an adolescent patient after cardiac surgery. Anesth Analg 2004; 98: 966-9.
Carvalho B, Dyer RA. Norepinephrine for spinal hypotension during cesarean delivery: another paradigm shift? Anesthesiology 2015; 122: 728-30.
Kinsella SM, Carvalho B, Dyer RA, Consensus Statement Collaborators, et al. International consensus statement on the management of hypotension with vasopressors during caesarean section under spinal anaesthesia. Anaesthesia 2018; 73: 71-92.
Author contributions
Imran Bhat contributed to study design; acquisition, analysis, and interpretation of data; and drafting the article. Virendra K. Arya contributed to all aspects of this manuscript, including study conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Banashree Mandal and Aveek Jayant contributed to study design; acquisition and interpretation of data; and drafting the article. Vikas Dutta contributed to analysis and interpretation of data and drafting the article. Sandeep Singh Rana contributed to acquisition of data and drafting the article.
Acknowledgements
We would like to thank Dr.Subrashis Guha Niyogi and Dr. Rajarajan Ganesan, PGIMER-Chandigarh India, for helping with editing the manuscript.
Conflict of interest
None.
Funding statement
Institutional resources PGIMER -Chandigarh India; no external funding
Editorial responsibility
This submission was handled by Dr. Philip M. Jones, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was presented in abstract form on October 21, 2017 at the annual meeting of the American Society of Anesthesiologists in Boston.
Rights and permissions
About this article
Cite this article
Bhat, I., Arya, V.K., Mandal, B. et al. Postoperative hemodynamics after high spinal block with or without intrathecal morphine in cardiac surgical patients: a randomized-controlled trial. Can J Anesth/J Can Anesth 68, 825–834 (2021). https://doi.org/10.1007/s12630-021-01937-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-021-01937-z