Abstract
Purpose
Perioperative complications of patients with idiopathic pulmonary fibrosis (IPF) are not well described. The aim of this study was to identify risk factors associated with adverse postoperative outcomes in IPF patients.
Methods
We performed a single-centre historical cohort study of adult patients with IPF who underwent surgery between 2008 and 2018. We analyzed the prognostic utility of select perioperative factors for postoperative acute exacerbation of IPF (AE-IPF), acute respiratory worsening (ARW), pneumonia, and 30-day and one-year mortality using univariable and multivariable regression analyses. To adjust for multiple interactions, the false discovery rate (Q value) was utilized to appropriately adjust P values and a Q value < 0.05 was considered to be significant.
Results
Two hundred and eighty-two patients were identified. After excluding emergency cases and bronchoscopies performed for active pneumonia, 14.2% of the cohort developed ARW that persisted > 24 hr after surgery, 5.0% had AE-IPF, and 9.2% were diagnosed with postoperative pneumonia within 30 days of surgery. The 30-day mortality was 6.0% and the one-year mortality was 14.9%. Preoperative home oxygen use (relative risk [RR], 2.70; 95% confidence interval [CI], 1.50 to 4.86; P < 0.001) and increasing surgical time (per 60 min) (RR, 1.03; 95% CI, 1.02 to 1.05; P < 0.001) were identified as independent risk factors for postoperative ARW.
Conclusions
In IPF patients, preoperative home oxygen requirement and increasing surgical time showed a strong relationship with postoperative ARW and may be useful markers for perioperative risk stratification. Facteurs de risque périopératoires des patients atteints de fibrose pulmonaire idiopathique : une étude de cohorte historique
Résumé
Objectif
Les complications périopératoires chez les patients atteints de fibrose pulmonaire idiopathique (FPI) ne sont pas bien décrites. L’objectif de cette étude était d’identifier les facteurs de risque associés aux devenirs postopératoires défavorables chez les patients atteints de FPI.
Méthode
Nous avons réalisé une étude de cohorte historique monocentrique portant sur des patients adultes atteints de FPI et ayant subi une chirurgie entre 2008 et 2018. Nous avons analysé l’utilité pronostique de facteurs périopératoires choisis pour l’exacerbation postopératoire aiguë de la FPI, la détérioration respiratoire aiguë, la pneumonie, et la mortalité à 30 jours et à un an à l’aide d’analyses de régression univariées et multivariées. Afin de tenir compte d’interactions multiples, le taux de fausses découvertes (valeur Q) a été utilisé pour ajuster adéquatement les valeurs P, et une valeur Q < 0,05 a été considérée significative.
Résultats
Deux cent quatre-vingt-deux patients ont été identifiés. Après avoir exclu les cas en urgence et les bronchoscopies réalisées lors de pneumonie active, 14,2 % des patients de la cohorte ont souffert d’une détérioration respiratoire aiguë qui a persisté > 24 h après la chirurgie, 5,0 % ont subi une exacerbation aiguë de la FPI, et 9,2 % ont reçu un diagnostic de pneumonie postopératoire dans les 30 jours suivant leur chirurgie. La mortalité à 30 jours était de 6,0 %, et la mortalité à un an de 14,9 %. L’utilisation préopératoire d’oxygène à domicile (risque relatif [RR], 2,70; intervalle de confiance [IC] 95 %, 1,50 à 4,86; P < 0,001) et l’augmentation du temps chirurgical (par tranche de 60 min) (RR, 1,03; IC 95 %, 1,02 à 1,05; P < 0,001) ont été identifiées comme des facteurs de risque indépendants de détérioration respiratoire aiguë en période postopératoire.
Conclusion
Chez les patients atteints de FPI, une forte association a été observée entre les besoins préopératoires en oxygène au domicile ainsi que l’augmentation du temps chirurgical et la détérioration respiratoire aiguë en période postopératoire; ces deux facteurs pourraient constituer des marqueurs utiles pour stratifier le risque en période périopératoire.
Similar content being viewed by others
Interstitial lung disease (ILD) constitutes a heterogeneous group of pulmonary conditions associated with a wide range of etiologies, but with a common pathway of fibrotic and inflammatory alterations to lung architecture.1,2 The prevalence of ILD is approximately 89/100,000 in males and 67/100,000 in females, and overall survival is better in females.3,4,5 Patients with ILD have considerably higher perioperative morbidity and mortality compared with non-ILD patients undergoing thoracic and non-thoracic surgery.6,7,8,9,10
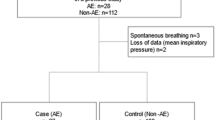
Patient selection flowchart. 1,933 surgical encounters in patients with the preoperative diagnosis of interstitial lung disease (ILD) were extracted, of which 1,247 were excluded for non-idiopathic pulmonary fibrosis (IPF) diagnoses such as sarcoidosis-related pulmonary fibrosis. Individual charts were analyzed to confirm either computed tomography (CT) evidence or surgical biopsy findings of IPF, which excluded an additional 33 patients. After excluding cases involving emergency procedures or pre-existing active pneumonia, and including only the most recent surgical encounter, our study cohort consisted of 282 patients.
Idiopathic pulmonary fibrosis (IPF) is the most common subgroup of ILD.2,11 The diagnosis of IPF comprises the highest overall mortality risk factor of ILD-related conditions.12,13 The overall mortality rate for IPF is on an uptrend, with an age-adjusted mortality increase of 28.4% in males and 41.3% in females.14 Perioperative outcomes in patients with the diagnosis of IPF are not comprehensively characterized in the literature. An increased risk for acute exacerbation of IPF (AE-IPF) has been shown in IPF patients undergoing diagnostic bronchoalveolar lavage.15 In addition, surgical lung biopsy for diagnosing IPF has a reported 2.1% risk of AE-IPF and a 30-day mortality rate of 5.1-7.1%.16,17,18 Furthermore, lung cancer patients with IPF have higher resection-related morbidity and mortality than those without IPF.19 The purpose of this study was to determine the preoperative risk factors associated with postoperative acute respiratory worsening (ARW), AE-IPF, postoperative pneumonia, and 30-day and one-year mortality in IPF patients and compare these outcomes in thoracic versus non-thoracic surgery.
Methods
The study (study # 00010125) was approved with a waiver of informed consent by the Penn State Health Milton S. Hershey Medical Center and Penn State College of Medicine Institutional Review Board. We performed a single-centre historical cohort study of IPF patients ≥ 18 yr of age undergoing surgery (or invasive procedures) over a ten-year period (May 2008 to May 2018) at Penn State Health Milton S. Hershey Medical Center.
Selection and description of participants
Inclusion criteria for surgical procedures included inpatient and outpatient surgical procedures as well as gastroenterology endoscopic procedures (e.g., colonoscopy, esophogastroduodenoscopy, and endoscopic retrograde cholangiopancreatography). We excluded patients with identified preoperative pneumonia and those undergoing emergency surgery. There were no protocol-related exclusion criteria for anesthetic technique. Nevertheless, as most imaging or image-guided procedures involved conscious sedation and had a high percentage of incomplete data, these were excluded from the cohort. Electronic medical records of the research subjects were manually reviewed, and the cohort refined to include only patients with the diagnosis of IPF confirmed by computed tomography (CT) imaging or analysis of surgical biopsy tissue.2,11
Measurements
Demographic information included age, sex, and American Society of Anesthesiologists physical status. We included select comorbidities previously categorized in the Elixhauser Comorbidity Index.20 In cases where patients underwent multiple surgical interventions, only the most recent encounter from the electronic data query was included for analysis. Comorbidities were identified by inclusion on the patient’s active problem list, admission note, or anesthesia history record. We selected hypertension, obstructive sleep apnea, obesity (body mass index > 30 kg·m−2, coronary artery disease, history of deep vein thrombosis or pulmonary embolism, diabetes, pulmonary hypertension, chronic kidney disease stage III or greater, heart failure, atrial fibrillation, chronic obstructive pulmonary disease, asthma, or cancer of any kind. In addition, we noted the presence of preoperative corticosteroid use (> 5 mg prednisone or equivalent/day), home oxygen use, and smoking status (active, former, or non-smoker). We extracted pulmonary function test (PFT) data up to one year prior to the procedure and documented forced vital capacity (FVC), forced expiratory volume, total lung capacity, and diffusing capacity of the lung for carbon monoxide (DLCO). Length of stay was calculated from admission and discharge dates. Intraoperative data including surgery/intervention type, airway management, surgery duration, blood transfusion, select medications, and intraoperative fluid administration were also abstracted.
The measured outcomes included AE-IPF, postoperative ARW, postoperative pneumonia, and 30-day and one-year mortality. Given the diagnostic complexity of AE-IPF, we limited identification of AE-IPF to physician diagnosis or new postoperative initiation of high-dose corticosteroid therapy (equivalent to prednisone > 20 mg/24-hr period) within 30 days of the surgical procedure.21,22 Acute respiratory worsening was quantified as requirement for endotracheal intubation, non-invasive ventilation strategy (positive airway pressure or high-flow nasal cannula), or augmented oxygen supplementation for more than 24 hr after the procedure, all of which are associated with increased in-hospital morbidity.23,24 Postoperative pneumonia was defined as a documented diagnosis of pneumonia or radiographic interpretation of pneumonia within 30 days of the surgical procedure. Thirty-day and one-year mortality were calculated with respect to the date of surgical procedure.
Statistical analysis
All variables were summarized prior to analysis to assess their distributions and check for errors. Histograms and normal probability plots were applied to continuous variables to determine normality. To adjust for multiple testing, the false discovery rate (FDR) method was used to adjust the P values for comparisons made with each outcome and are represented as Q values. A Q value of < 0.05 was accepted as the threshold of significance. Bivariate logistic regression was used to assess association between the outcome variables and the independent variables. Risk ratios were used to interpret the magnitude and direction of significant associations. A subset of the most significant variables for ARW and one-year mortality was used in a final multivariable model with a stepwise model selection for guidance within the limitation of the one-in-ten rule of thumb for predictors with logistic regression. The final model provided risk ratios adjusted for the other included variables. Risk ratios based on an approach using Poisson regression with a robust error variance were generated and used to interpret the magnitude and direction of significant associations. Interactions between predictors in the final model were tested, but none were found to be significant. The potential independent variables were checked for multicollinearity using variance inflation factor statistics, and the fit of the final model was assessed using Deviance, Pearson, and Hosmer-Lemeshow goodness-of-fit statistics. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
We identified 1,933 surgical encounters for patients with ILD by International Classification of Diseases 10th Revision coding. After excluding non-IPF diagnoses and cases without CT or surgical biopsy findings of IPF, preceding surgical encounters for the same patient, active pneumonia, and emergency cases, we identified 282 patients who met the inclusion criteria (Figure). Characteristics of the study population are presented in Tables 1 and 2. The mean age was 62.9 yr with an average of 2.7 co-morbid conditions per patient. The most common condition was hypertension (63%), followed by obesity (31%), coronary artery disease (28%), diabetes (22%), cancer (21%), and chronic obstructive pulmonary disease (21%). Over 50% of subjects were either active or former smokers, and 18% utilized continuous home oxygen therapy prior to surgery. Thoracic procedures constituted 51% of surgical interventions in our cohort. A majority of procedures (91%) were performed under general anesthesia. Airway management consisted of endotracheal tube (54%), supraglottic airway (laryngeal mask airway) (25%), or non-invasive airway (21%). Mean (standard deviation) surgical duration was 93 (97) min. Most patients were extubated at the conclusion of surgery (90%) and transferred to the Postanesthesia Care Unit (84%).
Univariate analyses of perioperative variables are summarized in Tables 3 and 4. In our cohort, we observed the following incidences: AE-IPF (n = 14; 5.0%), postoperative pneumonia (n = 26; 9.2%), ARW (n = 40; 14.2%), 30-day mortality (n = 17; 6.0%), and one-year mortality (n = 42; 14.9%). Of note, no differences were found between thoracic and non-thoracic cohorts in AE-IPF (relative risk [RR], 1.16; 95% confidence interval [CI], 0.34 to 4.00; Q = 0.99), ARW (RR, 0.87; 95% CI, 0.45 to 1.70; Q = 0.81), postoperative pneumonia (RR, 1.60; 95% CI, 0.74 to 3.46; Q = 0.90) and 30-day mortality (RR, 0.41; 95% CI, 0.12 to 1.39; Q = 0.26).
Multivariable analysis was performed for respiratory failure and one-year mortality since there was an inadequate number of outcome events to analyze AE-IPF, pneumonia, or 30-day mortality in this cohort. These findings are summarized in Table 5. Preoperative home oxygen use (RR, 2.70; 95% CI, 1.50 to 4.86; P < 0.001) and surgical time (per 60 min) (RR, 1.03; 95% CI, 1.02 to 1.05; P < 0.001) were significant predictors of postoperative ARW. Increasing age (per ten years) (RR, 1.50; 95% CI, 1.27 to 1.79; P < 0.001), former tobacco smoking status (RR, 2.44; 95% CI, 1.32 to 4.52; P = 0.004), preoperative oral steroid use (RR, 2.17; 95% CI, 1.34 to 3.51; P = 0.002) and absence of intraoperative dexamethasone administration (RR, 0.19; 95% CI, 0.06 to 0.59; P = 0.004) were associated with one-year mortality.
Discussion
This study is the first to associate preoperative home oxygen use with postoperative ARW in IPF patients. In addition, longer surgery duration was associated with ARW in our cohort. Our observation that preoperative home oxygen use is a harbinger of postoperative ARW provides the most clinically relevant and helpful variable for the perioperative risk stratification of IPF patients. This is important because, in patients with fibrotic ILD, admission with ARW has been associated with increased in-hospital and post-discharge mortality regardless of the type of underlying ILD etiology or type of acute respiratory decompensation.23 In our study cohort, diagnosis of ARW also increased hospital length of stay from a median of one to three days, adding to perioperative cost burden. Patients initiated on home oxygen usually have severe exercise limitation, exertional and/or rest dyspnea, and deteriorating quality of life indices, although the few studies examining its use have not found convincing benefit.25,26,27 Therefore, home oxygen use likely indicates progression of the patient’s ILD and is a marker of overall functional capacity. Furthermore, the incidence of pulmonary hypertension increases with IPF progression, shares similar clinical features with IPF progression, and has a 30-50% prevalence in severe IPF.28 We observed that only 11% of our overall study cohort, and 18% of patients on home oxygen, were diagnosed with pulmonary hypertension. It is highly plausible that in our cohort, undiagnosed (and unmanaged) pulmonary hypertension may have contributed to the strong relationship between home oxygen use and postoperative ARW. Thus, observing home oxygen use may reinforce the need for detailed preoperative risk stratification, emphasizing the search for right ventricular underperformance and pulmonary hypertension.
We found an incidence of 9.2% for postoperative pneumonia in IPF patients. This is much higher than that reported in non-IPF patients.29,30 Bacterial pneumonia is common in hospitalized IPF patients, with an incidence of 9.5% and an in-hospital mortality rate of 34%. Previous reports of postoperative pneumonia in ILD patients of all types showed a 3.9% incidence of postoperative pneumonia.8,31 These discordant observations may be related to the higher risk inherent to the ILD subpopulation of IPF, and efforts should be directed to identify modifiable risk factors for postoperative pneumonia in this fragile patient population.
Univariate analysis revealed no difference in our measured outcomes between thoracic vs non-thoracic surgery populations. Although this may be related to insufficient sample size given the wide confidence intervals, it bears mentioning that many of the current insights gained from studying thoracic surgery patients may be applicable to IPF patients undergoing non-thoracic surgery. For example, one study observed that higher oxygen concentrations, larger tidal volumes, and prolonged mechanical ventilation may be associated with a higher risk of acute exacerbation of IPF after thoracic surgery.32 Although these observations were made on a small cohort, they are congruent with the poor outcomes associated with mechanical ventilation in non-surgical ILD patients in the intensive care unit.33,34 Moreover, with thoracic surgery patients, higher intraoperative fluid administration has been linked to postoperative acute exacerbation of IPF.35 We found a strong association between increasing surgery duration (thus longer duration of mechanical ventilation, and, perhaps, more intravenous fluid administration) and ARW. The univariate analysis suggested a relationship between ARW and increased fluid administration and blood transfusion but was not supported by the multivariable analysis.
This study has limitations inherent to historical cohort studies. We attempted to limit selection bias by confirming IPF diagnosis with surgical biopsy or CT findings. Nevertheless, diagnosis of IPF is complex and we did not tabulate multidisciplinary confirmation of IPF.36 Secondly, we could not reliably stratify our study population based on the severity of IPF because of the complexities and controversies of ILD grading.37 Although it has been shown that an absolute decline in FVC > 10% or in DLCO > 15% over a six-month time period is strongly associated with disease progression in non-surgical ILD patients, this study was inadequately powered to assess relationships between PFT values and postoperative outcomes.38 Furthermore, we attempted to reduce conclusion error and control multiplicity (selection) effects by utilizing an FDR to adjust P values for multiple interactions. We acknowledge that mortality may be underestimated in our study because of inadequate follow-up. We emphasize that ILD encompasses a vast array of distinct subpopulations, which may differ from IPF patients with regard to various perioperative outcomes.
In summary, in IPF patients, preoperative home oxygen requirement and increasing surgical time showed a strong relationship with postoperative ARW. In this type of patient, home oxygen use and anticipated longer surgery may be useful markers for increased postoperative risk stratification. Postoperative pneumonia rates were comparatively high in IPF patients and further studies should be directed at identifying potential modifiable risk factors.
References
Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med 1981; 70: 542-68.
Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788-824.
Putman RK, Hatabu H, Araki T, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA 2016; 315: 672-81.
Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979-1991. An analysis of multiple-cause mortality data. Am J Respir Crit Care Med 1996; 153: 1548-52.
Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994; 150: 967-72.
Yuksel M, Ozyurtkan MO, Bostanci K, Ahiskali R, Kodalli N. Acute exacerbation of interstitial fibrosis after pulmonary resection. Ann Thorac Surg 2006; 82: 336-8.
Utz JP, Ryu JH, Douglas WW, et al. High short-term mortality following lung biopsy for usual interstitial pneumonia. Eur Respir J 2001; 17: 175-9.
Choi SM, Lee J, Park YS, et al. Postoperative pulmonary complications after surgery in patients with interstitial lung disease. Respiration 2014; 87: 287-93.
Kumar P, Goldstraw P, Yamada K, et al. Pulmonary fibrosis and lung cancer: risk and benefit analysis of pulmonary resection. J Thorac Cardiovasc Surg 2003; 125: 1321-7.
Tsubochi H, Shibano T, Endo S. Recommendations for perioperative management of lung cancer patients with comorbidities. Gen Thorac Cardiovasc Surg 2018; 66: 71-80.
Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733-48.
Thomeer MJ, Vansteenkiste J, Verbeken EK, Demedts M. Interstitial lung diseases: characteristics at diagnosis and mortality risk assessment. Respir Med 2004; 98: 567-73.
Bjoraker JA, Ryu JH, Edwin MK, et al. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 1998; 157: 199-203.
Olson AL, Swigris JJ, Lezotte DC, Norris JM, Wilson CG, Brown KK. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am J Respir Crit Care Med 2007; 176: 277-84.
Hiwatari N, Shimura S, Takishima T, Shirato K. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med 1994; 174: 379-86.
Kondoh Y, Taniguchi H, Kitaichi M, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med 2006; 100: 1753-9.
Lettieri CJ, Veerappan GR, Helman DL, Mulligan CR, Shorr AF. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005; 127: 1600-5.
Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am J Respir Crit Care Med 2016; 193: 1161-7.
Kawasaki H, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol 2002; 81: 33-7.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36: 8-27.
Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265-75.
Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med (Lausanne) 2017; DOI: https://doi.org/10.3389/fmed.2017.00176.
Moua T, Westerly BD, Dulohery MM, Daniels CE, Ryu JH, Lim KG. Patients with fibrotic interstitial lung disease hospitalized for acute respiratory worsening: a large cohort analysis. Chest 2016; 149: 1205-14.
Johannson KA, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis: a proposal. Curr Respir Care Rep 2013; 2: 233-40.
Visca D, Tsipouri V, Mori L, et al. Ambulatory oxygen in fibrotic lung disease (AmbOx): study protocol for a randomised controlled trial. Trials 2017; DOI: https://doi.org/10.1186/s13063-017-1912-9.
McDonald CF. Exercise desaturation and oxygen therapy in ILD and COPD: similarities, differences and therapeutic relevance. Respirology 2018; 23: 350-1.
Bell EC, Cox NS, Goh N, et al. Oxygen therapy for interstitial lung disease: a systematic review. Eur Respir Rev 2017; DOI: https://doi.org/10.1183/16000617.0080-2016.
Collum SD, Amione-Guerra J, Cruz-Solbes, et al. Pulmonary hypertension associated with idiopathic pulmonary fibrosis : current and future perspectives. Can Respir J 2017; DOI: https://doi.org/10.1155/2017/1430350.
Chughtai M, Gwam CU, Mohamed N, et al. The epidemiology and risk factors for postoperative pneumonia. J Clin Med Res 2017; 9: 466-75.
Chughtai M, Gwam CU, Khlopas A, et al. The incidence of postoperative pneumonia in various surgical subspecialties: a dual database analysis. Surg Technol Int 2017; 30: 45-51.
Oda K, Yatera K, Fujino Y, et al. Respiratory comorbidities and risk of mortality in hospitalized patients with idiopathic pulmonary fibrosis. Respir Investig 2018; 56: 64-71.
Sakamoto S, Homma S, Mun M, Fujii T, Kurosaki A, Yoshimura K. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med 2011; 50: 77-85.
Fernandez-Perez ER, Yilmaz M, Jenad H, et al. Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Chest 2008; 133: 1113-9.
Saydain G, Islam A, Afessa B, Ryu JH, Scott JP, Peters SG. Outcome of patients with idiopathic pulmonary fibrosis admitted to the intensive care unit. Am J Respir Crit Care Med 2002; 166: 839-42.
Mizuno Y, Iwata H, Shirahashi K, et al. The importance of intraoperative fluid balance for the prevention of postoperative acute exacerbation of idiopathic pulmonary fibrosis after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2012; 41: e161-5.
Quinn C, Wisse A, Manns ST. Clinical course and management of idiopathic pulmonary fibrosis. Multidiscip Respir Med 2019; DOI: https://doi.org/10.1186/s40248-019-0197-0.
Robbie H, Daccord C, Chua F, Devaraj A. Evaluating disease severity in idiopathic pulmonary fibrosis. Eur Respir Rev 2017; DOI: https://doi.org/10.1183/16000617.0051-2017.
Faverio P, De Giacomi F, Bonaiti G, et al. Management of chronic respiratory failure in interstitial lung diseases: overview and clinical insights. Int J Med Sci 2019; 16: 967-80.
Author contributions
All authors contributed to all aspects of this study, including generation of study concept, data acquisition, data analysis, and manuscript writing.All authors contributed to all aspects of this study, including generation of study concept, data acquisition, data analysis, and manuscript writing.
Disclosures
None.
Funding statement
Support provided from institutional and/or departmental sources.
Editorial responsibility
This submission was handled by Dr. Steven Backman, Associate Editor, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDowell, B.J., Karamchandani, K., Lehman, E.B. et al. Perioperative risk factors in patients with idiopathic pulmonary fibrosis: a historical cohort study. Can J Anesth/J Can Anesth 68, 81–91 (2021). https://doi.org/10.1007/s12630-020-01828-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01828-9