Abstract
Purpose
Post-craniotomy pain is a common clinical issue and its optimal management remains incompletely studied. Utilization of a regional scalp block has the potential advantage of reducing perioperative pain and opioid consumption, thereby facilitating optimal postoperative neurologic assessment. The purpose of this study was to assess the efficacy of regional scalp block on post-craniotomy pain and opioid consumption.
Methods
We performed a prospective randomized-controlled trial in adults scheduled to undergo elective supratentorial craniotomy under general anesthesia to assess the efficacy of postoperative bilateral scalp block with 0.5% bupivacaine with 1:200,000 epinephrine compared with placebo on postoperative pain and opioid consumption. The primary outcome was the visual analogue scale (VAS) for pain at 24 hr postoperatively.
Results
Eighty-nine patients were enrolled (n = 44 in block group; n = 45 in control group). There was no difference in the mean (standard deviation) VAS score at 24 hr postoperatively between the treatment group and the control group [31.2 (21.4) mm vs 23.0 (19.2) mm, respectively; mean difference, 6.6; 95% confidence interval, -2.3, 15.5; P = 0.15]. There was also no significant difference in postoperative opioid consumption. Distribution of individual VAS score and opioid consumption revealed that postoperative pain was highly variable following craniotomy. Time to hospital discharge was not different between treatment and placebo groups. No adverse events associated with scalp block were identified.
Conclusion
These data show that bilateral scalp blocks using bupivacaine with epinephrine did not reduce mean postoperative VAS score or overall opioid consumption at 24 hr nor the time-to-discharge from the postanesthesia care unit or from hospital.
Trial registration
www.ClinicalTrials.gov, NCT00972790; registered 9 September, 2009.
Résumé
Objectif
La douleur post-craniotomie est un problème clinique courant et sa prise en charge optimale n’a pas encore été exhaustivement étudiée. Le recours à un bloc régional du scalp comporte l’avantage potentiel de réduire la douleur périopératoire et la consommation d’opioïdes, facilitant ainsi une évaluation neurologique postopératoire optimale. L’objectif de cette étude était d’évaluer l’efficacité d’un bloc régional du scalp pour soulager la douleur post-craniotomie et réduire la consommation d’opioïdes.
Méthode
Nous avons réalisé une étude randomisée contrôlée prospective auprès d’adultes devant subir une craniotomie supratentorielle non urgente sous anesthésie générale afin d’évaluer l’efficacité d’un bloc bilatéral postopératoire du scalp réalisé avec de la bupivacaïne 0,5 % (1 : 200 000) comparativement à un placebo pour réduire la douleur postopératoire et la consommation d’opioïdes. Le critère d’évaluation principal était l’échelle visuelle analogique (EVA) pour les scores de douleur à 24 h postopératoires.
Résultats
Quatre-vingt-neuf patients ont été recrutés (n = 44 dans le groupe bloc; n = 45 dans le groupe témoin). Aucune différence n’a été observée dans le score moyen (écart type) sur l’EVA à 24 h postopératoires entre le groupe traitement et le groupe témoin [31,2 (21,4) mm vs 23,0 (19,2) mm, respectivement; différence moyenne, 6,6; intervalle de confiance 95 %, -2,3 à 15,5; P = 0,15]. Aucune différence significative n’a été observée dans la consommation postopératoire d’opioïdes non plus. La distribution des scores individuels de l’EVA et dans la consommation d’opioïdes a révélé que la douleur postopératoire était très variable après une craniotomie. Le délai jusqu’au congé de l’hôpital était similaire dans les deux groupes. Aucun événement indésirable associé au bloc du scalp n’a été identifié.
Conclusion
Ces données montrent qu’un bloc bilatéral du scalp à base de bupivacaïne et d’épinéphrine n’a pas réduit le score postopératoire moyen de l’EVA ni la consommation globale d’opioïdes à 24 h, ni le délai jusqu’au congé de la salle de réveil ou de l’hôpital.
Enregistrement de l’étude
www.ClinicalTrials.gov, NCT00972790; enregistrée le 9 septembre 2009.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The optimal postoperative analgesic management for patients undergoing craniotomy remains elusive. Clinicians have sought to minimize the deleterious hemodynamic responses of undertreated pain, while balancing undesired sedative effects of opioid-based analgesics, which could affect postoperative neurologic assessment and delay the early detection of postoperative complications.
The incidence of post-craniotomy pain is often underappreciated. A recent prospective study found that that up to 55% of patients had moderate or severe postoperative pain in the first 24 hr following craniotomy.1 Such a high incidence of moderate to severe pain is consistent with previous literature.2,3,4,5,6 The consequence of inadequate analgesia can be significant, as the arterial hypertension associated with undertreated post-craniotomy pain may cause cerebral hyperemia, edema, and hemorrhage.7,8 In addition, some patients proceed to develop long-term pain syndromes, with the incidence reaching as high as 25% in some studies.9,10,11,12
There remains a high degree of variability amongst clinicians in managing post-craniotomy pain, with a tendency for default treatment to be weak opioids such as codeine phosphate despite the fact that many neuroanesthesiologists believe that this form of treatment is unsatisfactory.13,14,15,16,17,18,19 In addition to the varied pharmacologic management, the century-old technique of “scalp block” during craniotomy continues to be used and studied as a peripheral nerve block that has potential benefit for patients undergoing craniotomy.20 In a 2011 qualitative systematic review of pain treatment after craniotomy, Hansen et al. reported that scalp blocks may provide analgesia for six hours after surgery but concluded that no firm recommendations on analgesic therapy could be given because of the small number of well performed randomized-controlled trials (RCTs).21
Few clinical trials have assessed the efficacy of local-regional anesthetic techniques in patients undergoing craniotomy. Nevertheless, different anesthetics and concentrations (ropivacaine 0.5%22 or 0.75%23,24; bupivacaine 0.25%25,26,27 or 0.375%23) different block timing (before22,25,26 or after23,24,27 surgery), different techniques (surgical wound infiltration23,25,26vs scalp nerve blocks22,24,27), make it impossible to meaningfully compare those studies. Moreover the sample size of those studies is relatively small. In addition, none of the above-mentioned studies used a standardized postoperative rescue analgesia technique.
The purpose of our study was to determine if scalp nerve blocks reduce postoperative pain score and improve clinical outcome in patients undergoing supratentorial craniotomy. We hypothesized that scalp nerve blocks performed at the end of surgery would reduce postoperative pain scores, opioid-related side-effects, as well as the time required for postanesthesia care unit (PACU) and hospital discharge.
Methods
Participants
The study protocol was approved (23 January 2008) by the St. Michael’s Hospital Research Ethics Board and was registered on clincaltrials.gov (NCT00972790; 9 September 2009). Written informed consent was obtained prior to study enrollment. Participants were recruited from St. Michael’s Hospital, a tertiary neurosurgical centre affiliated with the University of Toronto. We enrolled patients who were ≥ 18 yr of age, who were scheduled for elective supratentorial craniotomy, and who had an American Society of Anesthesiologists physical status I–III. We excluded patients with conditions contraindicating the use of the anesthesia maintenance protocol of this study, or with potential difficulties to discern postoperative pain specifically related to the surgery itself. Specific inclusion and exclusion criteria are outlined in eAppendix 1 (available as Electronic Supplementary Material [ESM]).
Randomization, stratification, and concealment of allocation
Patients were randomized in a 1:1 ratio in permuted blocks of two and four using a computer-generated list. Allocation information and instruction for the pharmacists preparing the solutions to be used for the blocks were placed in opaque sealed envelopes identified by a progressive number. On the day of the procedure, the envelopes were delivered to and opened by a pharmacist, who then prepared the study solution into syringes with identical appearances and delivered the syringes to the attending anesthesiologists in the operating rooms. Each syringe was labelled with a unique study number and an allocation code known only to the pharmacists.
Study investigators, enrolled patients, data analysts, and all members of the healthcare team and research team were blinded to treatment allocation. The pharmacists who were not involved in the conduct of the study or in the care of the patient were not blinded to group assignment.
Study intervention
A standardized anesthesia protocol was executed for all patients. Arterial waveform monitors and temperature probes were employed in addition to standard Canadian Anesthesiologists Society recommended monitoring.28 General anesthesia and tracheal intubation were achieved with fentanyl (1.5–3 µg·kg−1iv), propofol (2–3 mg·kg−1iv), rocuronium (0.6–1.2 mg·kg−1iv), and, at the discretion of the attending anesthesiologist, remifentanil (1–1.5 µg·kg−1iv). Anesthesia was maintained with sevoflurane (minimum alveolar concentration, 0.6–1.2) in O2 and air (fraction of inspired oxygen 0.40 or higher). Remifentanil (1–1.5 µg·kg−1iv) was given one minute before the placement of the pin head holder to prevent a hemodynamic reaction to the noxious stimulus, and a remifentanil infusion (0.0–0.5 µg·kg−1·min−1iv) was maintained until closure of the dura. The surgeons infiltrated the incision line and the pin sites with 1% lidocaine + epinephrine 1:200,000 before the placement of the pin head holder. Muscle relaxants were administered intraoperatively as needed and were reversed at the end of the surgery with neostigmine (0.04 mg·kg−1) and glycopyrrolate (0.006 mg·kg−1). Hydromorphone (0.4–1.2 mg iv) was titrated intraoperatively at the discretion of the attending anesthesiologists up to one hour prior to the end of surgery. No other intraoperative adjunct analgesics were given. Routine ondansetron 4 mg iv was given 30 min prior to the end of surgery as an emesis prophylaxis.
In the treatment group, bilateral scalp nerve blocks were performed using 20 mL of 0.5% bupivacaine with 1:200,000 epinephrine at the end of the procedure while patients were still under general anesthesia and in the head holders. The blocks were performed by the principal investigator or by the attending anesthesiologist or neurosurgeon according to the previously described technique (see eAppendix 2, available as ESM).29 In the control group, the patients received sham nerve blocks with 20 mL of saline with 1:200,000 epinephrine with identical techniques as that described for the treatment group.
Upon arrival to the PACU or intensive care unit (ICU), each patient received hydromorphone 0.1 mg iv every five minutes as needed (to a maximum of 2 mg) administered by a nurse until the patient was able to use a patient-controlled analgesia (PCA) pump. Postoperative pain was treated with hydromorphone PCA 0.1 mg bolus with a lockout interval of five minutes and a maximum of 6 mg in four hours in both study arms for at least 24 hr postoperatively. As per hospital policy, the decision to discontinue PCA, whether earlier or later than the first 24 hr, was made by the Acute Pain Services who were also blinded to study treatment allocation. For study purposes, we only examined PCA outcomes for the first 24 postoperative hours. Additionally, each patient received acetaminophen 650 mg (rectally or orally) every six hours. Once PCA was discontinued, each patient received acetaminophen 650 mg orally every six hours as needed, and hydromorphone 2–4 mg orally every three hours as needed.
Outcomes
The primary outcome was the postoperative visual analogue scale (VAS) for pain at 24 hr after the block was performed. This outcome measure was selected because the VAS has been shown to be linearly related to the perception of mild-to-moderate pain.30 Secondary outcomes included: 48 hr postoperative pooled VAS score; 24 hr postoperative total PCA hydromorphone consumption; 24 hr postoperative total hydromorphone demands and delivered doses; 24 hr and 48 hr incidence of nausea and vomiting; time to eligibility for discharge from PACU and from hospital; long-term pain as measured with numeric rating scale (NRS)31 on postoperative days 5, 30, and 60; and the Karnofsky Performance Score and Pain Treatment Satisfaction scale (PTSS) on postoperative day 5.
Data collection
Initial assessment included a standard preoperative assessment and study questionnaire. Patients were asked to report their pain using VAS at 30 min, one, two, four, eight, 12, 18, 24, and 48 hr, by a trained research coordinator or a trained bedside ICU nurses. Assessments for secondary outcomes occurred within the first 48 postoperative hours. Following patient discharge, a phone interview was conducted on the fifth, 30th, and 60th postoperative day.
Statistical analysis
Sample size was calculated based on data available in published studies.6,22,24,32 Assuming a two-sided α value of 0.05 and a β value of 0.1, we estimated that 38 patients in each group would be required to detect a 2.25-point difference in the VAS score, given an estimated VAS score of 4.5 in the control group with a standard deviation (SD) of 3 (50% reduction). To accommodate potential dropouts and the possibility that the VAS SD was different in our patients, we planned to recruit a total of 90 subjects. We were aware that this sample size may have not provided sufficient power to show a statistically significant difference between the two study arms for some of the secondary outcomes (PACU and hospital stay). Nevertheless, we considered them “exploratory outcomes”, that would provide a basis for a larger study should they show an evident trend.
Statistical analysis
All data are either presented as mean (SD) or as frequency and percentage (%), respectively. All analyses were conducted using the modified intention-to-treat principle. Descriptive statistics, including measures of central tendency and proportions, were performed on baseline and clinical characteristics of the study groups. A multiple linear regression model was utilized to adjust for differences in baseline characteristics between groups.
The VAS scores over time were analyzed using a linear mixed-effect model. A non-linear time trend was modelled using a cubic spline with three degrees of freedom as well as a time by treatment interaction. This model was utilized to obtain and assess differences in treatment effect at all time points. A student’s t test was used to assess differences in total PCA hydromorphone consumption, Karnofsky Performance Score, and pain scores from the PTSS. A Kaplan–Meier analysis was used to assess differences in time to eligibility for discharge from the PACU and hospital. An area-under-the-curve analysis was utilized to assess differences in pooled 48 hr VAS scores. A Chi-square test was utilized to assess differences in moderate to severe pain as scored by NRS on postoperative days 5, 30, and 60; and nausea occurrence at 24 and 48 hr. A Fisher’s exact test was utilized to assess vomiting at 24 and 48 hr. A P < 0.05 was considered statistically significant. Statistical analyses were performed with the R statistical package, version 3.5.1 (R Core Team, July 2018; www.r-project.org).
Results
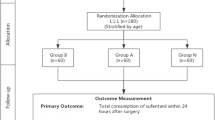
A total of 89 patients were enrolled in the study from March 2010 to October 2011, with 45 patients in the control group and 44 patients in the treatment group (Fig. 1). One patient in the control group and three in the treatment group did not receive the allocated treatment because of intraoperative complications. Since they remained intubated postoperatively the outcomes in those patients could not be assessed. Patient characteristics were similar as reported in Table 1. There was no apparent differences between groups except for a slight imbalance of the sex distribution and the VAS pain score at recruitment. This difference in VAS score was not observed at the preoperative assessment.
The mean (SD) VAS score at 24 hr was not different between the treatment group and the control group [31.2 (21.4) mm vs 23.0 (19.2) mm, respectively; mean difference, 6.6; 95% confidence interval [CI], -2.3, 15.5; P = 0.15) (Table 2) The total mean (SD) PCA hydromorphone consumption at 24 hours was also not different between two groups [4.0 (3.5) mg vs 3.2 (2.7) mg, respectively; mean difference, 0.8; 95% CI, -0.5, 2.2; P = 0.23).
The mean VAS scores at each time point are reported in Table 2. The assessment of the VAS scores over time performed by calculating the area-under-the-curve of the pooled 48 hr VAS scores did not show statistically significant difference between the two groups. Nevertheless, the plot of the VAS scores over time suggested that the VAS score had a non-linear trend over time and that this non-linear time trend differed between the two groups. A likelihood ratio test of the time by treatment interaction gave P < 0.001 (Fig. 2). This model showed the average predicted VAS scores based on the model (Fig. 2).
Mean VAS score in 48 hr fitted to a linear mixed-effect model where time was modelled using a cubic spline with three degrees of freedom and an interaction between treatment and the spline. A likelihood ratio test of the interaction resulted in a P value of P < 0.001. Dots represent the mean VAS scores. Shaded regions indicate the 95% confidence intervals for the spline curves. VAS = visual analogue scale
There was no significant difference in time-to-discharge from hospital and from PACU between the two groups (P = 0.43 and P = 0.50, respectively) (Table 2, Fig. 3). There was no significant difference in the Karnofsky Performance Score and in the PTSS at postoperative day 5 or in the presence of long-term pain as measured with NRS on postoperative day 5, 30, or 60 (Table 2 and Fig. 4). There was a high degree of variability between VAS score and opioid consumption at 24 hr, without any clear relationship between these parameters in individual patients from either placebo or scalp block treated patients (Fig. 5).
No adverse effects were observed in response to the application of scalp block with local anesthetic or placebo.
Discussion
Patients undergoing supratentorial craniotomy experience significant postoperative pain, and under-treatment of this pain may result in unfavourable cerebral hemodynamics and postoperative outcomes.1,7 In the current study, bilateral scalp blocks using bupivacaine with epinephrine did not reduce mean postoperative VAS score or overall opioid consumption at 24 hr. The data were fitted into a linear mixed-effect model (post hoc analysis). We observed a reduced mean VAS score during the early postoperative course prior to the 12-hr mark. The raw VAS score comparison indicates a difference in the relative VAS scores over time. In the first 12 postoperative hours, the VAS scores were higher in the control group than the treatment group. At 12 hr, there was no difference between groups and after the 12 hr time point the VAS appeared to be higher in the treatment group. Thus, there was a relative change in the VAS score between groups over time. In other words, there was a significant time-VAS score interaction (P < 0.001).
While some studies showed persistent analgesic benefits of scalp blocks up to and beyond 24 hr, our observation in the current study is consistent with a previous meta-analysis by Guilfoyle et al. showing efficacy of scalp blocks for post-craniotomy pain up to 12 hr postoperatively but not 24 hr.24,33,34 Our observation is also consistent with what is expected of the clinical duration of bupivacaine with epinephrine in the context of peripheral nerve block, especially since the scalp is a highly vascular area with rapid local anesthetic uptake.32,35
These findings are consistent with a 2013 meta-analysis of regional scalp block for post-craniotomy pain, which found reduced pain scores at one hour postoperatively. Nevertheless, they do not support the finding of an overall reduction in opioid requirements in the first 24 postoperative hours.33 In that publication, a subgroup analysis showed reduced postoperative pain scores for up to eight hours in patients who received preoperative scalp block, and up to 12 hr in those who received postoperative scalp block. They concluded that published RCTs of scalp block are small, heterogeneous, and of limited methodological quality, but that meta-analysis shows a consistent finding of reduced early postoperative pain, consistent with our findings. Interestingly, the one prospective RCT examining scalp incisional infiltration showed a reduction in persistent pain (56 vs 8%) and neuropathic pain (25 vs 4%) two months later.12
Contrary to previous studies, we failed to show a reduction in total opioid consumption at 24 hr in the current study.33 Nevertheless, it is important to point out the significant heterogeneity amongst the studies included in the meta-analysis. It is also not surprising that in our study nausea and vomiting (a common opioid-related side-effect) was not statistically significant different between the two groups. Of interest, we observed an increase in postoperative pain and opioid requirement in the second 12 hr after scalp block, possibly due to underutilization of opioid in the first 12 hr and rebound pain after the block resolved. These data are hypothesis deriving as the study was not powered to detect or assess pain outcomes at these time points.
Our prospective blinded RCT examined the effect of scalp blocks on persistent pain following supratentorial craniotomy beyond the acute postoperative period. Contrary to a prior study showing that incisional infiltration reduced persistent pain at two months following craniotomy, our study showed that scalp blocks, along with pin site and incisional infiltration, had no effect on the incidence of persistent pain following craniotomy as measured by NRS on postoperative days 5, 30, and 60.12 One important difference is that Batoz et al. used 0.75% ropivacaine for wound margin infiltration prior to scalp closure, while we used 1% lidocaine with epinephrine for pin site and incisional infiltration prior to incision, and 0.5% bupivacaine for the scalp block at the end of surgery. This observation provides directions for future studies to examine different protocols for preventing persistent surgical pain following craniotomy.
Limitations of our current study included that we were only able to show a pain score difference between the two groups when the mean postoperative VAS scores were fitted to a linear mixed-effect model over time. This may explain why we obtained different results than previous published RCTs; however, our larger sample size and estimate of power suggest this may be a true finding. An additional limitation is the relatively large VAS reduction (50%) considered for the sample size calculation. Although this estimate is quite large, we decided to take into consideration a value that would have clinical significance. In taking this approach, we preferred to accept the risk of a negative trial result rather than choosing a smaller effect size and reaching statistical significance but with little clinical significance. Future RCTs with a larger sample size may be required to further elucidate the acute benefits of scalp blocks on VAS scores, opioid consumption, and opioid-related side-effects during the early postoperative period. Another limitation is that the sample size of our study was not sufficiently powered to assess statistically significant differences in clinically relevant secondary outcomes, including PACU and hospital stay. Indeed, we failed to show a significant difference in time-to-discharge from hospital and from PACU between the two groups.
In conclusion, our study did not support that bilateral scalp blocks reduce mean postoperative VAS score at 24 hr, total opioid consumption at 24 hr, or time-to-discharge from PACU or from hospital. Further studies should specifically examine the effect of bilateral scalp blocks on pain scores, opioid consumption, and opioid-related side-effects during the early postoperative course.
References
Mordhorst C, Latz B, Kerz T, et al. Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol 2010; 22: 202-6.
Quiney N, Cooper R, Stoneham M, Walters F. Pain after craniotomy. A time for reappraisal? Br J Neurosurg 1996; 10: 295-9.
De Benedittis G, Lorenzetti A, Migliore M, Spagnoli D, Tiberio F, Villani RM. Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery 1996; 38: 466-9; discussion 469-70.
Graham AC, Reid MM, Andrews PJ. Perception of pain experienced and adequacy of analgesia following elective craniotomy. Anaesthesia 1999; 54: 814-5.
Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anesth 2007; 54: 544-8.
Gottschalk A, Berkow LC, Stevens RD, et al. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg 2007; 106: 210-6.
Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology 2000; 93: 48-54.
Bruder N, Pellissier D, Grillot P, Gouin F. Cerebral hyperemia during recovery from general anesthesia in neurosurgical patients. Anesth Analg 2002; 94: 650-4.
Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol 2010; 23: 551-7.
Kaur A, Selwa L, Fromes G, Ross DA. Persistent headache after supratentorial craniotomy. Neurosurgery 2000; 47: 633-6.
Gee JR, Ishaq Y, Vijayan N. Postcraniotomy headache. Headache 2003; 43: 276-8.
Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg 2009; 109: 240-4.
Klimek M, Ubben JF, Ammann J, Borner U, Klein J, Verbrugge SJ. Pain in neurosurgically treated patients: a prospective observational study. J Neurosurg 2006; 104: 350-9.
Jeffrey HM, Charlton P, Mellor DJ, Moss E, Vucevic M. Analgesia after intracranial surgery: a double-blind, prospective comparison of codeine and tramadol. Br J Anaesth 1999; 83: 245-9.
Stoneham MD, Walters FJ. Post-operative analgesia for craniotomy patients: current attitudes among neuroanaesthetists. Eur J Anaesthesiol 1995; 12: 571-5.
Vadivelu N, Kai AM, Tran D, Kodumudi G, Legler A, Ayrian E. Options for perioperative pain management in neurosurgery. J Pain Res 2016; 9: 37-47.
Roberts GC. Post-craniotomy analgesia: current practices in British neurosurgical centres–a survey of post-craniotomy analgesic practices. Eur J Anaesthesiol 2005; 22: 328-32.
Leslie K, Williams DL. Postoperative pain, nausea and vomiting in neurosurgical patients. Curr Opin Anaesthesiol 2005; 18: 461-5.
Verchere E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol 2002; 14: 96-101.
Osborn I, Sebeo J. “Scalp block” during craniotomy: a classic technique revisited. J Neurosurg Anesthesiol 2010; 22: 187-94.
Hansen MS, Brennum J, Moltke FB, Dahl JB. Pain treatment after craniotomy: where is the (procedure-specific) evidence? A qualitative systematic review. Eur J Anaesthesiol 2011; 28: 821-9.
Gazoni FM, Pouratian N, Nemergut EC. Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: a pilot study. J Neurosurg 2008; 109: 44-9.
Law-Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol 2005; 17: 139-43.
Nguyen A, Girard F, Boudreault D, et al. Scalp nerve blocks decrease the severity of pain after craniotomy. Anesth Analg 2001; 93: 1272-6.
Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg 1998; 87: 579-82.
Biswas BK, Bithal PK. Preincision 0.25% bupivacaine scalp infiltration and postcraniotomy pain: a randomized double-blind, placebo-controlled study. J Neurosurg Anesthesiol 2003; 15: 234-9.
Ayoub C, Girard F, Boudreault D, Chouinard P, Ruel M, Moumdjian R. A comparison between scalp nerve block and morphine for transitional analgesia after remifentanil-based anesthesia in neurosurgery. Anesth Analg 2006; 103: 1237-40.
Dobson G, Chow L, Flexman A, et al. Guidelines to the practice of anesthesia - revised edition 2019. Can J Anesth 2019; 66: 75-108.
Pinosky ML, Fishman RL, Reeves ST, et al. The effect of bupivacaine skull block on the hemodynamic response to craniotomy. Anesth Analg 1996; 83: 1256-61.
Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg 1999; 89: 1517-20.
McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988; 18: 1007-19.
Bala I, Gupta B, Bhardwaj N, Ghai B, Khosla VK. Effect of scalp block on postoperative pain relief in craniotomy patients. Anaesth Intensive Care 2006; 34: 224-7.
Guilfoyle MR, Helmy A, Duane D, Hutchinson PJ. Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth Analg 2013; 116: 1093-102.
Hwang JY, Bang JS, Oh CW, et al. Effect of scalp blocks with levobupivacaine on recovery profiles after craniotomy for aneurysm clipping: a randomized, double-blind, and controlled study. World Neurosurg 2015; 83: 108-13.
Costello TG, Cormack JR, Hoy C, et al. Plasma ropivacaine levels following scalp block for awake craniotomy. J Neurosurg Anesthesiol 2004; 16: 147-50.
Author contributions
Andrea Rigamonti, Marco M. Garavaglia, Gregory M.T. Hare, and C. David Mazer contributed substantially to the conception and design of the study, and acquisition, analysis, and interpretation of data. Kan Ma, Nikhil Mistry, and Kevin Thorpe contributed substantially to the study design, and to the analysis and interpretation of data. Charmagne Crescini contributed substantially to the acquisition and analysis of data. Michael Cusimano and Sunit Das contributed substantially to the conception and design of the study, and interpretation of data. All authors contributed to drafting, revising, and adding important intellectual content to the manuscript.
Acknowledgements
Thank you to Dr. Andreas Laupacis for his support with protocol design.
Conflicts of interest
Dr. Hare has received funding from Forest Research Inc. for basic science studies (2010–11) and salary support from Johnson & Johnson Inc., (2012–14) for his role in the St. Michael’s Hospital Center of Excellence for Patient Blood Management.
Funding statement
This study was supported by the Physicians Services Incorporated Grant (PSI 09-22, PI Andrea Rigamonti). Drs. Rigamonti, Hare, and Mazer were supported by the University of Toronto, Department of Anesthesia Merit Awards.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rigamonti, A., Garavaglia, M.M., Ma, K. et al. Effect of bilateral scalp nerve blocks on postoperative pain and discharge times in patients undergoing supratentorial craniotomy and general anesthesia: a randomized-controlled trial. Can J Anesth/J Can Anesth 67, 452–461 (2020). https://doi.org/10.1007/s12630-019-01558-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01558-7