Abstract
Background
Major depressive disorder (MDD) is a common and debilitating condition that can be challenging to treat. Electroconvulsive therapy (ECT) is currently the therapeutic gold standard for treatment-resistant MDD. We tested our hypothesis that ketamine-based anesthesia for ECT results in superior improvement in treatment-resistant MDD outcomes compared with propofol-based anesthesia.
Methods
Patients with treatment-resistant MDD were enrolled in a randomized clinical trial with assignment to ketamine- or propofol-based anesthesia arms. Using a modified intention-to-treat analysis, we compared the median number of ECT treatments required to achieve a 50% reduction (primary outcome) and a score ≤ 10 (secondary outcome) on the Montgomery-Asberg depression rating scale (MADRS) between anesthesia groups.
Results
The study was terminated as significant results were found after the first planned interim analysis with 12 patients in each of the ketamine (intervention) and propofol (control) groups. All ketamine patients achieved at least a 50% MADRS reduction after a median of two ECT treatments whereas ten propofol patients (83%) achieved the same outcome after a median of four ECT treatments. All ketamine patients and seven propofol patients (58%) achieved MDD remission (MADRS ≤ 10). Log rank tests showed that both time-to-50% reduction and remission differed significantly between groups. Adverse events and recovery time were similar between groups.
Conclusions
In this early-terminated small-sized study, ketamine-based anesthesia compared with propofol-based anesthesia provided response and remission after fewer ECT sessions.
Trial registration
www.clinicaltrials.gov (NCT01935115). Registered 4 September 2013.
Résumé
Contexte
Le trouble dépressif majeur (TDM) est une affection fréquente et invalidante qui peut être difficile à traiter. L’électro-convulsivothérapie (ECT) est actuellement l’option de choix pour les TDM résistant au traitement pharmacologique. Nous avons testé l’hypothèse qu’une anesthésie à base de kétamine pour l’ECT contribuerait à de meilleurs résultats dans le traitement du TDM résistant qu’une anesthésie à base de propofol.
Méthodes
Des patients atteints de TDM résistant au traitement ont été inclus dans cet essai clinique randomisé pour recevoir une anesthésie à base de kétamine ou une anesthésie à base de propofol. Nous avons comparé au moyen d’une analyse en intention-de-traiter modifiée le nombre médian d’ECT requis pour obtenir une réduction de 50% (critère d’évaluation principal) et un score ≤ 10 (critère d’évaluation secondaire) sur l’échelle d’évaluation de la dépression de Montgomery-Asberg (MADRS) entre les groupes d’anesthésies.
Résultats
L’étude a été arrêtée de façon précoce, car des résultats significatifs ont été trouvés à la première analyse intérimaire prévue avec 12 patients dans chaque groupe : kétamine (groupe interventionnel) et propofol (groupe témoin). Tous les patients du groupe kétamine ont obtenu une réduction d’au moins 50% sur l’échelle MADRS après un nombre médian de deux ECT, alors que seulement dix patients du groupe propofol (83%) parvenaient au même résultat après un nombre médian de 4 traitements par ECT. Tous les patients du groupe kétamine et sept patients du groupe propofol (58%) ont obtenu une rémission du TDM (MADRS ≤ 10). Des tests du rang logarithmique ont montré que le délai d’atteinte de la réduction de 50% et le délai d’obtention de la rémission étaient tous deux significativement différents entre les groupes. Les événements indésirables et les temps de récupération ont été semblables entre les deux groupes.
Conclusions
Dans cette étude de petite taille arrêtée précocement, l’anesthésie à base de kétamine a entraîné une réponse et une rémission après moins de séances d’ECT qu’une anesthésie à base de propofol.
Enregistrement de l’essai clinique
www.ClinicalTrials.gov (NCT01935115). Enregistré le 4 septembre 2013.
Similar content being viewed by others
Major depressive disorder (MDD) is a common psychiatric illness with an estimated lifetime prevalence in excess of 15%.1,2 The burden of MDD is staggering with the World Health Organization estimating it to be the leading cause of disability in developed countries for those aged 15-44.2 Oral antidepressants are the most common treatment. Nevertheless, partial efficacy, delayed onset of action for weeks, and side effects of these medications are important limitations.3 Further, the United States Federal Food and Drug Administration has warned that certain patient groups are at increased risk for morbidity and suicidal ideation after initiation or dose change of oral antidepressants.4 Treatment-resistant depression (TRD) is suggested when symptoms of MDD persist after at least two trials of antidepressants from different pharmacologic classes fail to produce significant clinical improvement.5 The gold standard therapy for TRD is currently electroconvulsive therapy (ECT).6 Electroconvulsive therapy has rapid antidepressant effects beginning with the first week of treatment.7 Nevertheless, ECT has limitations, as do oral antidepressants; only a proportion of patients respond to ECT, and both cognitive side effects and relapse after ECT are common.8 It has been shown that in patients suffering from MDD, suicidal thoughts persisted in 62% after one week of thrice-weekly ECT treatment and in 39% after two weeks.9 In addition, the complexity and costs of ECT are drawbacks.
Novel pharmaceutical agents with rapid antidepressant effects are a new and promising area in MDD research.10,11,12 Emerging literature suggests that glutamatergic modulating agents, in particular ketamine, can induce rapid improvement in depression in both preclinical models and humans.13,14 Given anesthesiologists’ extensive experience with ketamine and its reported benefits in MDD, we sought to test our hypothesis that ketamine-based anesthesia is superior to propofol-based anesthesia for patients suffering from TRD undergoing ECT.
Methods
Study design
The study protocol was approved by the local Biomedical Research Ethics Board (Saskatoon, SK, Canada; Bio13-156) and was registered at www.clinicaltrials.gov (NCT01935115). This double-blinded clinical trial randomly allocated patients referred for ECT treatment to either ketamine- or propofol-based anesthesia arms. Patients in the ketamine arm (intervention) initially received intravenous ketamine 0.75 mg·kg−1, remifentanil 1 µg·kg−1, and succinylcholine 0.75 mg·kg−1. Patients in the propofol (control) arm initially received intravenous propofol 1 mg·kg−1 and identical doses of remifentanil and succinylcholine. Based on patients’ anesthetic response, the attending anesthesiologist was given freedom to vary the dose of remifentanil and succinylcholine as well as administer additional propofol to achieve safe and acceptable anesthetic conditions. No supplementation of ketamine with propofol was necessary during this study. The study drugs were prepared beforehand by the clinical trials pharmacist who also carried out the randomization. A random sequence of “1’s” and “2’s” was generated in blocks of size 24 as implemented with Research Randomizer (www.randomizer.org). This sequence was kept in a binder that was accessible only to pharmacists. Upon enrolment, each participant’s allocation was kept in a sealed envelope for drug preparation.
Ketamine was mixed with Intralipid® by the hospital pharmacy to make its appearance identical to propofol. Anesthetic care included standard monitoring including oximetry, electrocardiography, non-invasive blood pressure, and end-tidal carbon dioxide as well as standard post-anesthesia recovery care.
As per the local health region’s care standard, all patients were enrolled for eight ECT sessions scheduled at two or three sessions/week. Unilateral or bilateral electrode placement and ECT therapy parameters were determined by the attending psychiatrist. The psychiatrist had the option to discontinue ECT treatment if the patient achieved remission or ECT was ineffective based on clinical judgement. After each ECT procedure, patients were monitored by a nurse who was blind to anesthetic agent and adverse events such as delirium, hallucinations, and agitation. These were recorded in standard case report forms.
The protocol called for an interim analysis to be performed by a statistician who was blinded to group allocation after 20 and after 40 patients. An independent safety committee was informed of the results of the interim analysis including side effects and complications and had the option to adjust the drug dosage or to discontinue the trial.
Participants
Eligible patients referred for ECT from October 2013 to February 2016 by attending psychiatrists were approached for voluntary enrolment. Participation eligibility criteria included: age greater than 17 yr, diagnosis of TRD, a Montgomery-Asberg Depression Rating Scale (MADRS) score of at least 20, and ability to provide informed consent. Exclusion criteria were as follows: American Society of Anesthesiology physical status score greater than 3, an implanted medical device, major cardiovascular disease (including untreated hypertension (HTN), major respiratory disease, cerebrovascular disease, intracranial HTN (including glaucoma), seizures, a diagnosis of schizoaffective disorder, pregnancy, or allergy to any of the study drugs. Involuntary status was the main reason for exclusion. Screening for eligibility and obtaining informed consent to participate were performed by a member of the research team.
Psychiatric measurement tools
The MADRS is a frequently used ten-item scale that assesses severity of depression over the past week. It emphasizes psychologic symptoms of depression rather than physical symptoms that are more easily affected by other drugs such as sedatives.15 The MADRS was administered before the first ECT (at baseline), one day after each ECT session, and 30 days after the final ECT session. During and after the ECT treatment course, the time interval instructions of the MADRS were modified to assess depression occurring since the last ECT treatment. The timings for ECT treatment and MADRS assessments were regular because psychiatrists administered ECT from 8:00 to 10:00 a.m. during weekdays. Research assistants visited the patients the next day for MADRS assessments. Please refer to the supplementary Excel file for the assessment times.
The 19-item version of the Clinician Administered Dissociative States Scale (CADSS) was used to measure distortions in perception and experience 30 min after each ECT treatment.16 The short form of the Affective Lability Scale (ALS-18) was also administered before the first ECT treatment (baseline) and 30 days after the last ECT administration. It is an 18-item scale that retrospectively measures sudden mood changes over the past week.17
Psychiatric outcomes
Two related outcomes are typically reported in ECT trials for major depression: remission and response. Response is generally defined as a 50% reduction in depression scores using a patient- or clinician-rated instrument.18,19 Remission is defined as achieving a score ≤ 10 on the MADRS.20,21 Accordingly, we defined the study’s primary outcome as the number of ECT sessions required to achieve a 50% reduction from the baseline MADRS score. Our secondary outcomes were: the number of ECT sessions required to achieve remission (MADRS ≤ 10), the proportion of depressed patients (MADRS > 20) at 30 days after the last ECT session, and the change in ALS scores from baseline to 30 days after the last ECT session.
Anesthetic outcomes
Hemodynamic data were recorded during anesthesia. As per local practice non-invasive blood pressure was measured immediately prior to induction of anesthesia, every 2.5 min while anesthetized, and every 15 min after emergence until discharge from the ECT suite. Increases or decreases in baseline systolic blood pressure at any point during the anesthetic care were categorically recorded as minimal change (20-50 mmHg from baseline) and significant change (more than 50 mmHg from baseline). Adverse hemodynamic changes requiring any pharmacologic therapy treatment were also recorded. The decision to treat hemodynamics changes was left to the discretion of the anesthesiologist. Patient-reported post-ECT outcomes included any events of nausea or vomiting and headache. Postanesthetic recovery nurse-reported outcomes included the subjective presence or absence of emergence agitation, brief delirium (any less than one hour after emergence), prolonged delirium (any present longer than one hour after emergence), brief hallucinations (any less than one hour after emergence), and prolonged hallucinations (any present longer than one hour after emergence). The time from anesthesia induction to post-anesthesia care discharge readiness was recorded.
Statistical analysis
We were unsure how to perform sample size calculation based on the primary outcome, so we calculated it based on the hypothesized proportion of patients responding in each arm. A required sample size of 56 patients (28 in each arm) was calculated with the estimate that after eight ECT sessions, 40% of ketamine and 20% of propofol patients would achieve a 50% reduction in MADRS score at a power of 80% and one-sided alpha of 0.05. Please refer to our supplementary Word file for power calculation details. Since the number of ECT sessions depends in part on the particular patients enrolled, we based our sample size calculation on expected response rates as suggested by the literature10,22 in combination with clinical experience. The choice of a one-sided alpha was motivated by previous literature suggesting ketamine’s superiority over propofol.23,24 To allow for attrition, we planned to recruit 36 patients per arm. A patient achieving improvement or remission prior to the eighth ECT session was considered as having achieved an outcome and not lost to attrition. For the interim analysis, we followed the Pocock adjustment method to control for type I error.25 This required an adjusted alpha of 0.0294 to achieve significance.
Our primary outcome was analyzed by a modified intention-to-treat analysis. To be eligible for analysis, patients needed to receive at least one ECT treatment.20 We used Kaplan-Meier survival analysis to compare proportions of patients responding and remitting in each treatment arm. From these models, the median number of treatments to a 50% MADRS reduction and to remission were then calculated. In these univariate models, drug was the sole predictor and the log rank test was used to compare the survivor functions. Subsequently, we created age- and sex-adjusted discrete time survival models using complementary log-log regression. Fisher’s exact test was used to compare the proportion of patients experiencing depression recurrence (MADRS > 20) 30 days after the final ECT session. We compared ALS scores at baseline with those at day 30 using linear mixed modelling. Clinician Administered Dissociative States Scale scores were compared by drug group at each ECT session using linear mixed modelling. For fixed effects, we entered time and treatment plus their interaction. We allowed for a random intercept for each patient experiencing depression recurrence (MADRS > 20) 30 days after the final ECT session.
Results
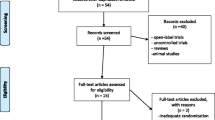
Twenty-seven subjects were recruited from mid-September 2013 to the end of November 2015. Following the first scheduled interim analysis, we reported our results to the research ethics board and were advised to stop the trial because clinical equipoise no longer held. Consequently, our interim analysis became our final results. Of 28 patients referred by psychiatrists, 27 participated in the study with 14 assigned to the ketamine arm and 13 to the propofol arm (Fig. 1). The demographic and clinical characteristics of participants were similar between drug groups (Table 1). Various measures related to ECT delivery are summarized in Table 2.
Primary outcome
All patients in the ketamine arm achieved a 50% MADRS reduction compared with 10 (83%) in the propofol arm. Patients allocated to ketamine had a median (interquartile range [IQR]) number of 2 [1-4] ECT treatments to a 50% MADRS reduction. By comparison, patients allocated to propofol had a median of 4 treatments [2-7] for the same outcome. The log rank test showed a statistically significant difference in survivor functions between groups (χ2 = 6.22, P = 0.01) (Fig. 2), thereby supporting the superiority of ketamine over propofol, after Pocock adjustment.
Boxplot of the Montgomery-Asberg depression rating scale (MADRS) scores by electroconvulsive therapy (ECT) session and anesthetic used. Numbers on the top row of the x-axis indicate the ECT session number. On the bottom row of the x-axis, “K” stands for ketamine (pink bars) and “P” stands for propofol (blue bars)
Secondary outcomes
Similarly, all patients in the ketamine arm achieved remission compared with 7 (58%) in the propofol arm. Ketamine patients had a median count of 3 treatments to remission [1-4] compared with 7 treatments [2 to not estimable] for propofol. The log rank test also showed a difference in the groups’ survivor functions (χ2 = 6.25, P = 0.01) (Fig. 3). Multivariate survival analysis showed that patients in the ketamine arm were more than twice as likely to achieve a 50% reduction in baseline MADRS (hazard ratio [HR]: 3.20, 95% confidence interval [CI]: 2.00 to 5.13) and were also twice as likely to achieve remission (HR: 3.67, 95% CI: 2.13 to 6.32) compared with the propofol arm. At 30-day follow-up, each treatment arm had a single patient relapse (MADRS > 20) (P = 0.77). Interestingly, the patient assigned to the ketamine arm remitted but relapsed, while the propofol arm patient did not remit during ECT treatment. Linear mixed modelling showed a significant decrease in ALS-SF scores at 30-day follow-up (B = -8.03, standard error (SE) = 3.23), but the change did not differ by drug group (P = 0.55).
Number of electroconvulsive therapy (ECT) sessions to a 50% Montgomery-Asberg depression rating scale (MADRS) score reduction (left panel) and to remission (MADRS score ≤ 10) (right panel). X-axis: Session number with zero indicates baseline. Y-axis: proportion of patients who have not achieved the primary outcome
Adverse events and patient recovery times
Mean [standard deviation (SD)] CADSS scores between the ketamine and propofol groups were similar across ECT sessions. For both groups, there was a decrease in CADSS scores with an increased number of ECT treatments (B = -0.57, SE: 0.74) (Table 3). Time from anesthesia induction to discharge readiness was similar between groups, 63.5 (18.2) vs 63.3 (15.8) min in the ketamine arm and propofol arm, respectively (P = 0.94). The rates of other adverse events were similar between groups (see Table 4).
Discussion
In this small-size study terminated early, ketamine- compared with propofol-based anesthesia for the provision of ECT provided: 1) faster improvement of depressive symptoms (50% reduction was attained after two ECT treatments with ketamine anesthesia vs four ECT treatments with propofol anesthesia, respectively), 2) fewer treatments to achieve disease remission (four ECT treatments in the ketamine arm vs seven ECT treatments in the propofol arm), and 3) similar 30-day remission rates. In our trial, we did not observe a difference between the groups in psychiatric or anesthetic adverse events; however, the sample size was small and our trial was not powered to detect differences in adverse effects.
The strengths of this study include the recruitment of patients with TRD depression, randomized patient allocation, and intention-to-treat data analysis. The patient, anesthesiologist, psychiatrist administering the ECT, recovery nurses, and research assistant rater were blind to the anesthetic agent used. No supplementary drugs were required. The study was done in a functioning psychiatric unit with patients recommended for ECT, who gave informed consent for the administration of ECT for the treatment of TRD. All of these patients were being treated with anti-depressant and mood-stabilizing drugs before and concurrent with ECT.
Our results are consistent with previous investigations on the antidepressant effects of ketamine. Several studies have shown that a single intravenous infusion of ketamine has a robust and rapid antidepressant effect on patients with uni- and bipolar depression.10,20,26 One to three doses per week over two to four weeks maintain improvement over the course of treatment and for approximately two additional weeks with the response rate varying from 70-91.6% after six doses of ketamine for TRD.11,20 An additional study has suggested that ketamine without ECT may be comparable to ECT with thiopental anesthesia.27 Furthermore, ketamine seems effective in reducing the symptom of suicidal thoughts in major depression.10,12
One meta-analysis of five studies using ketamine as part of anesthesia for ECT concluded a lack of clinical efficacy and increased likelihood of confusion.28 In four of these studies, ketamine was combined with another anesthetic induction agent, either propofol or thiopental. Only one study used ketamine alone, and that study was clearly positive for ketamine after two treatments.29 Another meta-analysis including 13 studies (four involving ECT, nine without ECT) found that ketamine was effective when used alone and in conjunction with ECT.10 It is possible that other sedative anesthetic agents used with ketamine such as propofol, barbiturates, or benzodiazepines may suppress seizures and reduce the efficacy of ketamine and ECT.30 Other reasons for different results might be differences in electrode placement and stimulus parameters as well as dosing of ketamine.30 Another recent double-blind study did not find a difference in depression outcomes in patients receiving ECT with either ketamine- or propofol-based anesthesia.31 This study differed from ours in that patients were not selected for TRD and the doses of ketamine and propofol were higher. Our study concurs with the results of a recent study of patients with TRD treated with ECT using anesthesia based on 1) ketamine, 2) ketamine plus propofol, and 3) propofol.24 In their study, the ketamine only group had the best outcomes, the propofol only group the worst, and ketamine plus propofol indistinguishable from the ketamine only group.
Despite the concern of “ketamine emergence” short-term dissociative symptoms following ketamine-based anesthesia, these symptoms have been inconsistently observed.20,30,31 Similar to several other studies, we used a relatively small dose of ketamine and no important dissociation was observed during recovery from anesthesia.29,30 Similarly, memory impairment was less frequently observed with ketamine anesthesia.29,30 It is possible that ECT reduces the dissociative effect of ketamine.29
The exact mechanism of action of ECT has yet to be determined.32 Regardless of the mechanism, the basics involve induction of seizure activity by the application of electricity to the scalp, with the resultant seizure in some way required for antidepressant activity.33 This association has lead clinicians to conclude that the duration of seizure is an important determinant of ECT efficacy. It may be the case that ECT-induced seizures of less than 15 sec are less effective; otherwise the bulk of the contemporary evidence suggests seizure length is not associated with ECT efficacy.33,34 This literature suggests that the longer motor seizure duration in the ketamine group in our study was not contributory to the observed differences in depression outcomes as duration of seizures in both groups exceeded 15 sec.
There are several postulated mechanisms for the antidepressant effects of ketamine. Although an in-depth discussion is beyond the scope of this article, an excellent recent review on the topic has been published.35 Other N-methyl-D-aspartate (NMDA) antagonists do not seem to produce the same antidepressant effects as ketamine, suggesting that its antidepressant properties may not be mediated via NMDA pathways. Also, it has been shown that ketamine’s antidepressant effect involves activation of alpha amino-3-hydroxy -5-methyl-4-isoxzole propionic acid (AMPA) receptors either directly or via metabolism to (2R, 6S)-HNK (hydroxynorketamine). It is postulated that the end result of AMPA activation increases brain-derived neurotrophic factor, thereby increasing synaptogenesis between neurons, a process thought to be central to depression recovery.36 This study had several limitations, the most notable of which were the small sample size (n = 24) and the decision by the ethical board to terminate the study. Our intended sample size was 72 patients, but the results of the first planned interim analysis led the board to discontinue the trial. In addition, our study was powered to detect a difference in the proportion of responders in each arm and not the number of treatments required for a response as such. This deviated from our published protocol in ClinicalTrials.gov. The confirmatory evidence for the efficacy of ketamine from other studies is strong, although not uniformly positive. We did not assess whether the patients were uni- or bipolar, but the difference may be a matter of degree.37 We also did not assess for psychotic symptoms, but all patients were able to give informed consent so psychotic symptoms were not prominent. The use of propofol for ECT may be considered a suboptimal comparator to ketamine as some consider methohexital to be the drug of choice for ECT.38 Numerous subsequent publications have shown similar outcomes whether methohexital or propofol was used to provide anesthesia for ECT.39,40,41 It is possible that the mixture of ketamine with Intralipid® somehow changed the potency of ketamine and altered responses might be observed if the same dose of ketamine were administered without Intralipid®. Although there is a paucity of literature directly addressing this potential issue, several avenues of investigation suggest this should not be of concern. First, a study using ketamine mixed with Intralipid® has been published with results in keeping with the expected dose-appropriate response of ketamine alone.33 Second, propofol is formulated in a lipid emulsion with the same composition as Intralipid. Many published studies describe using the combination of propofol and ketamine with good clinical effect.33,42,43,44,45,46,47 Further, a chemical analysis of the mixture of propofol and ketamine showed the chemical stability of both drugs in the mixture.48 A final design limitation was the non-standardized ECT electrode placement, which may have introduced bias into the results. The location of electrode placement was left to the judgement of the attending psychiatrist although this followed published guidelines.49 Finally, the distribution of electrode placement was similar between groups suggesting electrode placement did not contribute to the observed differences in outcomes.
Given that ketamine increases most hemodynamic parameters it is possible that adverse cardiovascular complications may occur with its administration.50 A detailed assessment of the hemodynamic response to treatment was not a primary objective and usual clinical monitoring of these brief treatments51 was all that was specified in the protocol. A significant change in blood pressure might have been missed but resulted in no difference in patient outcome or in the rates of treatment of hyper- or hypotension. Further, both groups received remifentanil, which may have blunted an otherwise more robust hemodynamic response experienced by those in the ketamine group. Such findings have not been observed in previously published literature studying ketamine alone for provision of ECT, suggesting this is not a major concern.29
Our results provide further evidence that ketamine-based anesthesia has the potential to reduce the number of ECT treatments required for remission of TRD. These encouraging results should be retested in a larger clinical trial, with a robust assessment of possible adverse effects including hemodynamic changes and their associated sequelae. This reduction promises to hasten patient recovery, decrease the risks of repeated anesthesia, decrease memory impairment and morbidity from ECT treatment, decrease the duration and cost of inpatient stays, and justify further research on novel treatments for depression.
References
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593-602.
World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008. Available from URL: https://www.fda.gov/NewsEvents/Testimony/ucm113265.htm/https://www.fda.gov/NewsEvents/Testimony/ucm113265.htm (accessed January 2018).
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5: e45.
U.S. Department of Health and Human Services. U.S. Food and Drug Administration. Anti-depressant Drug Use in Pediatric Populations - 2004. Available from URL: https://wayback.archive-it.org/7993/20170723044254/https://www.fda.gov/NewsEvents/Testimony/ucm113265.htm (accessed January 2018).
Berlim MT, Turecki G. Definition, assessment, and staging of treatment-resistant refractory major depression: a review of current concepts and methods. Can J Psychiatry 2007; 52: 46-54.
Uk ECT. Review Group. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003; 361: 799-808.
Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med 1993; 328: 839-46.
Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA 2001; 285: 1299-307.
Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry 2005; 162: 977-82.
Fond G, Loundou A, Rabu C, et al. Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 2014; 231: 3663-76.
Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134-42.
Reinstatler L, Youssef NA. Ketamine as a potential treatment for suicidal ideation: a systematic review of the literature. Drugs R D 2015; 15: 37-43.
Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 2015: CD011612.
McCloud TL, Caddy C, Jochim J, et al. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev 2015; 9: CD011611.
Lam RW, Michalak EE, Swinson RP. Assessment scales in depression, mania and anxiety. London, UK: Taylor and Francis; 2005 .
Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11: 125-36.
Oliver MNI, Simons JS. The affective lability scales: Development of a short-form measure. Pers Indiv Differ 2004; 37: 1279-88.
Jarventausta K, Chrapek W, Kampman O, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: a randomized pilot study. J ECT 2013; 29: 158-61.
Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in Depression: A Meta-Analytic Review. Journal of ECT 2004; 20: 13-20.
Singh JB, Fedgchin M, Daly EJ, et al. A double-blind, randomized, placebo-controlled, dose-frequency study of intravenous ketamine in patients with treatment-resistant depression. Am J Psychiatry 2016; 173: 816-26.
Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. J Psychiatr Res 2004; 38: 577-82.
Shelton RC, Osuntokun O, Heinloth AN, Corya SA. Therapeutic options for treatment-resistant depression. CNS Drugs 2010; 24: 131-61.
Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: comparing ketamine and propofol anesthesia. J ECT 2010; 26: 223-7.
Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT 2012; 28: 128-32.
Pocock SJ. Clinical Trials - A Practical Approach. Chichester: John Wiley & Sons; 1983 .
Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856-64.
Ghasemi M, Kazemi MH, Yoosefi A, et al. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res 2014; 215: 355-61.
McGirr A, Berlim MT, Bond DJ, et al. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J Psychiatr Res 2015; 62: 23-30.
Yoosefi A, Sepehri AS, Kargar M, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: a randomized, double-blind study. J ECT 2014; 30: 15-21.
Zhong X, He H, Zhang C, et al. Mood and neuropsychological effects of different doses of ketamine in electroconvulsive therapy for treatment-resistant depression. J Affect Disord 2016; 201: 124-30.
Fernie G, Currie J, Perrin JS, et al. Ketamine as the anaesthetic for electroconvulsive therapy: the KANECT randomised controlled trial. Br J Psychiatry 2017; 210: 422-8.
Fosse R, Read J. Electroconvulsive treatment: hypotheses about mechanisms of action. Front Psychiatry 2013; 4: 94.
Shah A, Mosdossy G, McLeod S, Lehnhardt K, Peddle M, Rieder M. A blinded, randomized controlled trial to evaluate ketamine/propofol versus ketamine alone for procedural sedation in children. Ann Emerg Med 2011; 57(425-33): e2.
Lalla FR, Milroy T. The current status of seizure duration in the practice of electroconvulsive therapy. Can J Psychiatry 1996; 41: 299-304.
Newport DJ, Carpenter LL, McDonald WM, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry 2015; 172: 950-66.
Zanos P, Moaddel R, Morris PJ, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533: 481-6.
Balbuena L, Baetz M, Bowen RC. The dimensional structure of cycling mood disorders. Psychiatry Res 2015; 228: 289-94.
Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg 2002; 94: 1351-64.
Geretsegger C, Nickel M, Judendorfer B, Rochowanski E, Novak E, Aichhorn W. Propofol and methohexital as anesthetic agents for electroconvulsive therapy: a randomized, double-blind comparison of electroconvulsive therapy seizure quality, therapeutic efficacy, and cognitive performance. J ECT 2007; 23: 239-43.
Kirkby KC, Beckett WG, Matters RM, King TE. Comparison of propofol and methohexitone in anaesthesia for ECT: effect on seizure duration and outcome. Aust N Z J Psychiatry 1995; 29: 299-303.
Vaidya PV, Anderson EL, Bobb A, Pulia K, Jayaram G, Reti I. A within-subject comparison of propofol and methohexital anesthesia for electroconvulsive therapy. J ECT 2012; 28: 14-9.
Andolfatto G, Willman E. A prospective case series of pediatric procedural sedation and analgesia in the emergency department using single-syringe ketamine-propofol combination (ketofol). Acad Emerg Med 2010; 17: 194-201.
Andolfatto G, Willman E. A prospective case series of single-syringe ketamine-propofol (ketofol) for emergency department procedural sedation and analgesia in adults. Acad Emerg Med 2011; 18: 237-45.
David H, Shipp J. A randomized controlled trial of ketamine/propofol versus propofol alone for emergency department procedural sedation. Ann Emerg Med 2011; 57: 435-41.
Jalili M, Bahreini M, Doosti-Irani A, Masoomi R, Arbab M, Mirfazaelian H. Ketamine-propofol combination (ketofol) vs propofol for procedural sedation and analgesia: systematic review and meta-analysis. Am J Emerg Med 2016; 34: 558-69.
Sharieff GQ, Trocinski DR, Kanegaye JT, Fisher B, Harley JR. Ketamine-propofol combination sedation for fracture reduction in the pediatric emergency department. Pediatr Emerg Care 2007; 23: 881-4.
Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med 2007; 49: 23-30.
Donnelly RF, Willman E, Andolfatto G. Stability of ketamine-propofol mixtures for procedural sedation and analgesia in the emergency department. Can J Hosp Pharm 2008; 61: 426-30.
Mankad MV, Beyer JL, Weiner RD, Krystal AD. Clinical Manual of Electroconvulsive Therapy. 1st ed. Washington, DC: American Psychiatric Publighing; 2010 .
Flood P, Rathmell JP, Shafer S. Stoelting’s Pharmacology and Physiology in Anesthetic Practice. 5th ed. Philadelphia: Wolters Kluwer Health; 2015 .
Dobson G, Chong M, Chow L, et al. Guidelines to the practice of anesthesia - revised edition 2017. Can J Anesth 2017; 64: 65-91.
Acknowledgements
We gratefully acknowledge the assistance of Dr. Dennis Lawson and Dr Jennifer O’Brien of the University of Saskatchewan. We also acknowledge the Departments of Anesthesiology, Perioperative Medicine and Pain Management and Department of Psychiatry, University of Saskatchewan, in facilitating this study. Finally, we acknowledge the support of the Saskatoon Health Region and College of Medicine, University of Saskatchewan. Detailed information regarding estimation of sample size and individual Montgomery Asberg Depression Rating Scale scores are provided as Electronic Supplementary Material.
Conflicts of interest
Commercial or non-commercial affiliations that are or may be perceived to be a conflict of interest with the work for each author, and any other associations, such as consultancies: No author has any commercial or other affiliations that are, or may be perceived to be, a conflict of interest.
Editorial responsibility
This submission was handled by Dr. Gregory L. Bryson, Deputy Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
The manuscript is the collaborative work of seven authors. Jonathan J. Gamble, Henry Bi, Rudy Bowen, Lloyd Balbuena, and Renuk Prasad conceptualized the study and provided theoretical guidance in interpretation. Rohan Sanjanwala, Henry Bi, and Grahme Weisgerber acquired the data. Lloyd Balbuena and Rohan Sanjanwala analyzed the data. Jonathan J. Gamble wrote the initial draft and all seven authors revised the manuscript. All of the authors approved the manuscript as submitted.
Funding
This study received support from the Schulman Research Award (University of Saskatchewan) and the Royal University Hospital Foundation (Saskatoon, Saskatchewan).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gamble, J.J., Bi, H., Bowen, R. et al. Ketamine-based anesthesia improves electroconvulsive therapy outcomes: a randomized-controlled study. Can J Anesth/J Can Anesth 65, 636–646 (2018). https://doi.org/10.1007/s12630-018-1088-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-018-1088-0