Abstract
Purpose
This randomized trial aimed to validate a new method for brachial plexus blockade, i.e., targeted intracluster injection supraclavicular block (TII SCB), by comparing it with ultrasound-guided axillary block (AXB). We hypothesized that TII SCB would result in a shorter total anesthesia-related time.
Methods
Forty patients undergoing upper limb surgery were randomized to ultrasound-guided TII SCB (n = 20) or AXB (n = 20). In the TII SCB group, we deposited 16 mL of lidocaine 1.5% with epinephrine 5 µg·mL−1 into the largest neural cluster (i.e., brachial plexus trunks/divisions). Subsequently, an additional 16 mL was divided into equal aliquots and injected inside each satellite cluster. In the AXB group, 5.5 mL were deposited around the musculocutaneous nerve and 23.5 mL were injected at the 6 o’clock position of the axillary artery. The main outcome for comparison between the two groups was the total anesthesia-related time (defined as the sum of block performance and onset times). We also recorded the number of needle passes, procedural pain, and complications (vascular puncture, paresthesia).
Results
The TII SCB method provided a quicker mean (SD) onset time compared with the AXB group [9.5 (5.8) min vs 18.9 (6.1) min; mean difference, −9.5 min; 99% CI, −14.7 to −4.2; P < 0.001] and a shorter mean (SD) total anesthesia-related time [20.1 (5.0) min vs 27.2 (6.5) min; mean difference, −7.0 min; 95% CI, −10.9 to −3.1; P = 0.001]. There were no intergroup differences in terms of success rate (95%), procedural pain, vascular puncture and paresthesia. The AXB group displayed a faster performance time [8.2 (1.6) min vs 10.6 (2.6) min; P = 0.001] with fewer median [interquartile range] needle passes (3 [2-6] vs 5 [4-8]; P < 0.001).
Conclusion
Ultrasound-guided TII SCB provides a quicker onset and a shorter total anesthesia-related time than ultrasound-guided AXB.
Résumé
Objectif
Cette étude randomisée visait à valider une nouvelle méthode de bloc du plexus brachial, c’est-à-dire un bloc supraclaviculaire par injection ciblée intraplexique (TII SCB - targeted intracluster injection supraclavicular block), en la comparant au bloc axillaire échoguidé (AXB - ultrasound-guided axillary block). Nous avons émis l’hypothèse que le TII SCB entraînerait un temps d’anesthésie total plus court.
Méthodes
Quarante patients subissant une chirurgie du membre supérieur ont été randomisés pour avoir un TII SCB (n = 20) ou un AXB (n = 20) échoguidé. Dans le groupe TII SCB, nous avons injecté 16 mL de lidocaïne à 1,5 % avec 5 µg·mL−1 d’épinéphrine dans le plus grand groupe nerveux (c’est-à-dire, les troncs et divisions du plexus brachial). Nous avons ensuite divisé 16 mL supplémentaires en aliquotes égales et les avons injectées dans chaque groupe satellite. Le groupe AXB a reçu une injection de 5,5 mL autour du nerf musculo-cutané et une injection de 23,5 mL en position « 6 heures » par rapport à l’artère axillaire. Le principal critère d’évaluation pour la comparaison entre les deux groupes était le temps total lié à l’anesthésie (défini par le temps total de la réalisation du bloc et du délai d’apparition de l’effet anesthésique). Nous avons également consigné le nombre de passages d’aiguilles, la douleur liée à la procédure et les complications (ponction vasculaire, paresthésies).
Résultats
La méthode du TII SCB a entraîné un délai moyen (ÉT) d’apparition de la réponse plus rapide que la méthode AXB (9,5 [5,8] minutes contre 18,9 [6,1] minutes; différence moyenne 9,5 minutes; IC à 99 %, −14,7 à −4,2; P < 0,001]) et un temps total moyen (ÉT) lié à l’anesthésie plus court (20,1 [5,0] minutes contre 27,2 [6,5] minutes; différence moyenne 7,0 minutes; IC à 95 %, −10,9 à −3,1; P = 0,001). Il n’y a pas eu de différences entre les groupes pour ce qui concerne le taux de succès (95 %), la douleur de la procédure, les ponctions vasculaires et les paresthésies. Le groupe AXB a affiché un temps de réalisation plus court (8,2 [1,6] minutes contre 10,6 [2,6] minutes; P = 0,001] avec un moins grand nombre médian (intervalle interquartile) de passages d’aiguille (3 [2-6] contre 5 [4-8]; P = 0,001).
Conclusion
Le TII SCB échoguidé entraîne une apparition plus rapide et un temps total lié à l’anesthésie plus court que l’AXB échoguidé.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recently, we have described a new targeted intracluster injection (TII) technique for ultrasound (US)-guided supraclavicular brachial plexus blockade (SCB) whereby local anesthetic (LA) agents are deposited inside the main as well as the satellite neural clusters (i.e., trunks/divisions of the brachial plexus).1 Compared to standard US-guided SCB,2,3 we have shown that the TII technique resulted in a shorter total anesthesia-related time (i.e., the sum of block performance time and onset time to a minimal sensorimotor composite score of 14 out of 16 points) in 90 subjects undergoing elective or semi-elective (fracture repair) upper limb surgery.1 In order to validate US-guided TII SCB, we also compared it with US-guided infraclavicular blocks in a similar study population of 64 subjects.4 Again, the TII SCB provided a shorter total anesthesia-related time.
In the present trial, we sought further validation of TII SCB by comparing it with the remaining major approach for brachial plexus blockade, US-guided axillary block (AXB). We hypothesized that US-guided TII SCB would yield a shorter total anesthesia-related time than AXB.
Methods
Our Ethics Committee (McGill University Health Centre, Montreal, QC, Canada) approved this study (November 2014), and 40 subjects undergoing elective or semi-elective (fracture repair) surgery of the forearm, wrist, or hand (Fig. 1) provided written informed consent. Exclusion criteria included age < 18 or > 80 yr, inability to consent to the study, American Society of Anesthesiologists physical status > III, body mass index < 20 or > 35 kg·m−2, preexisting neuropathy, coagulopathy, hepatic or renal failure, allergy to LA, pregnancy, and prior surgery in the axilla or supraclavicular fossa.
CONSORT diagram of patient flow through the study. Onset and total anesthesia-related times could not be recorded for patients with minimal composite scores < 14 points at 30 min. Nevertheless, all other study variables, i.e., imaging/needling/performance times, number of needle passes, number of clusters (SCB group), procedural pain, operator’s experience level, adverse events (vascular puncture/paresthesia), incidence of Horner’s syndrome, sensorimotor block of individual nerves, and successful surgical anesthesia, were recorded for these subjects. AXB = axillary brachial plexus block; TII SCB = targeted intracluster injection supraclavicular brachial plexus block
Upon arrival in the induction room, all patients received supplemental oxygen, standard monitoring,5 and intravenous premedication (midazolam 0.03 mg·kg−1 and fentanyl 0.6 μg·kg−1). Using sealed envelopes and a computer-generated sequence of random numbers, subjects were randomized to either US-guided TII SCB or AXB. Residents, fellows, or attending anesthesiologists performed all blocks under the supervision of the senior coauthor (D.Q.H.T.). For both approaches, operators were considered experts or trainees if they had performed > 60 or ≤ 60 blocks, respectively, prior to the commencement of the study.6 The US device (Sonosite M-Turbo, Bothell, WA, USA), 6–13 MHz linear probe, 5-cm 22-gauge block needle (StimuQuick Echo, Arrow International Inc, Reading, PA, USA) and LA (32 mL and 29 mL of lidocaine 1.5% with epinephrine 5 μg/mL for SCB and AXB, respectively) used were identical in all cases.
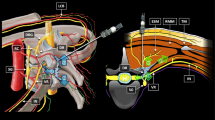
The TII SCB was carried out according to a previously described technique.1,4 Briefly, the supraclavicular fossa was scanned in a sterile fashion in search of a short-axis view of the subclavian artery. The main and satellite neural clusters were identified. After local skin infiltration (3 mL of lidocaine 1.5%) using an in-plane technique and a lateral-to-medial direction, the block needle was initially advanced until its tip was positioned inside the largest (main) cluster. Sixteen millilitres of the LA solution were injected in this location. Subsequently, an additional 16 mL were partitioned into equal aliquots and deposited inside each satellite cluster. For instance, if two, three, or four satellite clusters were identified, aliquots of 8, 5, 3, and 4 mL were used, respectively.
The US-guided AXB was also carried out according to a previously described technique.7 The US probe was applied in a sterile fashion in the axilla in order to obtain a short-axis view of the musculocutaneous nerve and axillary artery. After local skin infiltration (3 mL of lidocaine 1.5%), the block needle was initially advanced towards the musculocutaneous nerve where 5.5 mL of LA were deposited. The needle was then advanced until its tip was positioned just dorsal to the artery, and 23.5 mL of LA were then injected in this location. A “silhouette sign” (i.e., blurring of the arterial wall due to superimposed anechoic blood and anechoic LA) was sought to ensure proximity between artery and needle tip.7
For both techniques, we did not use hydrodissection; however, operators were allowed a small volume (< 1 mL) of normal saline to confirm successful positioning of the needle tip at the desired target (AXB: musculocutaneous nerve or 6 o’clock position of the axillary artery; TII SCB: main or satellite cluster).
For both groups, the investigator supervising the block recorded the imaging time, needling time, number of needle passes, number of clusters (TII SCB group), complications (vascular puncture and paresthesia), and procedural pain using a 11–point scale (0 = no pain; 10 = worst imaginable pain). Imaging time was defined as the temporal interval from the first US contact to the acquisition of a satisfactory image. Needling time was defined from the start of initial local skin infiltration until the end of LA injection through the 5-cm block needle. Thus, performance time was equal to the sum of the imaging and needling times. For the number of needle passes, initial needle insertion counted as the first pass, and any subsequent needle advancement preceded by a retraction of ≥ 1 cm was defined as an additional pass.8
Upon completion of the LA injection, an investigator blinded to group assignment assessed the sensory and motor block of the musculocutaneous, radial, median, and ulnar nerves every five minutes for 30 min using a three-point scale: 0 = no block; 1 = partial block; 2 = complete block.1,2,4,7 For each of the four nerves, partial and complete sensory block were defined as an inability to feel cold and light touch, respectively, whereas partial and complete motor block were defined as paresis and paralysis, respectively. Sensory blockade of the musculocutaneous, median, radial, and ulnar nerves was evaluated on the forearm (lateral aspect), thumb (volar aspect), hand (dorsolateral aspect), and fifth finger (volar aspect), respectively.1,2,4,7 Motor blockade of the musculocutaneous, radial, median, and ulnar nerves was assessed with elbow flexion, thumb abduction, thumb opposition, and thumb adduction, respectively.1,2,4,7 The maximal composite score was 16 points. The patient was considered ready for surgery upon reaching a score of ≥ 14 points, thereby defining the onset time. Therefore, the total anesthesia-related time was the sum of performance and onset times. We have used an identical scale in our previous studies.1,2,4,7 If the composite score was < 14 points after the last sensorimotor block assessment (at 30 min), the patient was transferred to the operating room for the start of the surgery. For these subjects, we did not record an onset time but did assess for the presence of surgical anesthesia (Fig. 1). Before leaving the induction room, the blinded observer recorded the incidence of Horner’s syndrome as well as the patient’s anthropometric data.
A blinded observer was responsible for assessing surgical anesthesia, which was defined as successful completion of surgery without the need for rescue blocks, supplemental LA infiltration, intravenous narcotics, or general anesthesia.1,2,4,7 Nevertheless, in the event of anxiety (self reported by patients or determined by the primary anesthesiologists), subjects received a propofol infusion (25-80 μg·kg−1·min−1) that allowed maintained response to verbal stimulus. In case of pain during surgery, the block was considered a failure and patients received rescue blocks, LA infiltration, intravenous narcotics, or general anesthesia.
During the initial block procedure, US-guided AXB and SCB catheters (StimuCath, Teleflex Medical, Research Triangle Park, NC, USA) were inserted for postoperative pain management. Axillary and SCB catheter tips were positioned dorsal to the axillary artery and at the “corner pocket” (i.e., outside of neural clusters), respectively. Placement of perineural catheters did not impact the recording of study data since it was carried out during the five-minute interval between the end of LA injection through the 22G block needle and the first sensorimotor assessment of the block. No LA or saline was injected during perineural catheter placement (3-4 cm beyond the needle tip). Furthermore, procedure-related pain and occurrence of paresthesia were assessed prior to catheter insertion.1 No additional LA was administered via these catheters until completion of the surgical procedure.
One week after the surgery, a blinded investigator contacted all study subjects to inquire about any complications such as residual numbness/paresthesia and/or motor deficits.
Statistical analysis
Our previous experience with US-guided TII SCB and AXB demonstrated mean (SD) total times of 18.2 (6.1) min and 27.1 (7.4) min, respectively.4,7 In anticipation of a similar 30% reduction in total time with TII SCB compared to AXB, approximately 16 subjects per group were required for a statistical power of 0.90 and a type I error of 0.025. By definition, onset and total times can be computed only for patients who attain a minimum of 14 points at 30 min. Because 90% of subjects achieved such scores in our previous studies,2,9 18 patients per group were needed to compensate for those who would not reach 14 points. We enrolled 40 subjects to account for potential patient dropout.
Time-related variables were analyzed with Welch’s test (Student’s t test for unequal variances). Normality was assessed using the Shapiro-Wilk test, and in the case of non-normal data (imaging and onset times), the significance level was reduced to P < 0.01 and the 99% confidence interval was presented. Ordinal data were analyzed using the Mann-Whitney U test, and Fisher’s exact test was employed for categorical data. All other P values were two-sided and values < 0.05 were considered significant unless otherwise specified. Statistical analysis was carried out using SPSS® version 20 statistical software (IBM, Armonk, NY, USA).
Results
Both groups were comparable in terms of demographics and surgical procedures (Table 1). The mean (SD) onset time was faster in the TII SCB group compared with the AXB group [9.5 (5.8) min vs 18.9 (6.1) min, respectively; mean difference, −9.5; 99% CI, −14.7 to −4.2; P < 0.001]. In terms of our primary endpoint, the mean (SD) total anesthesia-related time was also shorter in the TII SCB group compared with the AXB group [20.1 (5.0) min vs 27.2 (6.5) min, respectively; mean difference, −7.0; 95% CI, −10.9 to −3.1; P = 0.001] (Table 2). The AXB group necessitated fewer median (interquartile range [IQR]) needle passes than the SCB group (3 [2-6] vs 5 [4-8], respectively; P < 0.001) as well as shorter mean (SD) needling time [7.4 (1.7) min vs 9.9 (2.6) min, respectively; P = 0.001] and mean (SD) performance time [8.2 (1.6) min vs 10.6 (2.6) min, respectively; P = 0.001]. No intergroup differences were found in terms of overall success rate (95%), need for intraoperative sedation (90-100% of patients), complications (i.e., vascular puncture and paresthesia), and procedural pain. However the TII SCB group displayed a higher rate of Horner’s syndrome compared with the AXB group (45% vs 0%, respectively; P = 0.014) (Table 2).
In the TII SCB group, the faster overall onset time was reflected by higher proportions of patients achieving minimal composite block scores of 14 points during the first 15 min (Fig. 2). This could be explained by the fact that complete sensory/motor block of the radial and median nerves occurred more often with SCB during the first 15 min (all P < 0.028). Moreover, there was a higher incidence of ulnar motor block in the SCB group during the first 20 min (all P < 0.013). There were no intergroup differences in terms of sensory and motor block for the musculocutaneous nerve (Fig 3 and 4).
Percentage of patients with sensory anesthesia (score of 2) according to time in the cutaneous distributions of the radial, median, ulnar and musculocutaneous nerves. Absolute count values are provided inside each column. AXB = axillary brachial plexus block; SCB = supraclavicular brachial plexus block
At one week, patient follow-up revealed no persistent sensory or motor deficits in either group.
Discussion
In this randomized trial, we compared US-guided TII SCB and AXB and observed that, despite similar rates of successful surgical anesthesia, TII SCB resulted in a shorter total anesthesia-related time. Interestingly, these results appear to contradict our previous findings where we also compared US-guided SCB and AXB (as well as infraclavicular blocks) in 120 patients undergoing upper limb surgery and found similar total anesthesia-related times (23.1-25.5 min).10 However differences and variations in techniques preclude parallels between the two trials. For example, in our earlier trial, for SCB, we injected the entire LA volume (35 mL) at the “corner pocket” (i.e. intersection of the first rib and subclavian artery),10 whereas the contemporary TII technique used in the present study fragmented the LA volume in order to accurately target individual clusters. Furthermore, for AXB, we performed a four-injection technique in the prior trial,10 whereas the current study employed the recently described double-injection perivascular technique.7
Choosing different LA volumes for the 2 groups may appear counterintuitive. Patients in the SCB group benefited from 32 mL; in contrast, subjects randomized to AXB were given 29 mL. The 3 mL-difference might constitute a limitation. However we selected the respective volumes because they represent the documented MEV90s (i.e., the minimum effective LA volumes in 90% of subjects) for US-guided SCB and AXB.2,9 A universal volume of 32 mL might have favoured subjects randomized to AXB; conversely, 29 mL may have underdosed the SCB group. Thus, using demonstrated (but different) MEV90s allowed the levelling of success rates in order to isolate the primary research variable (total anesthesia-related time). Nevertheless, we recognize that 32 mL constitutes the MEV90 for two-injection SCB2 and not TII SCB. Since the MEV90 has not been elucidated for the latter, 32 mL represent its best estimate.
The different numbers of injections (two for AXB and multiple for TII SCB) may seem problematic. With the TII SCB technique, we injected LA into all neural clusters. In contrast, the AXB targeted only the musculocutaneous nerve; median/radial/ulnar nerve block was achieved through passive diffusion of LA deposited dorsal to the axillary artery. One could question the choice of such perivascular technique for AXB over a more targeted perineural technique whereby each of the four terminal nerves is methodically identified and anesthetized. However, in a previous trial,7 we compared perivascular and perineural US-guided AXB and observed that both methods resulted in similar success rates and total anesthesia-related times. Because of a shorter performance time and fewer needle passes, the perivascular method for AXB provided greater ease of performance.7 Since our main purpose was to validate a new method for brachial plexus block (TII SCB), the control group had to employ the best evidence-based technique. Thus, by selecting the double-injection perivascular technique for AXB, we sought to compare the experimental group (TII SCB) with an optimal control group.
The implication of intracluster injection remains controversial and warrants some discussion. In 2009, Bigeleisen et al.11 equated positioning the needle tip inside a cluster with intraneural placement. However in a recent editorial, Franco12 argued that “penetrating the prevertebral fascia during an interscalene or supraclavicular block, for example, does not constitute intraneural injection.” Our cumulative clinical experience stemming from the current and previous trials1,4 reveals no case of motor deficit or numbness exceeding one month and seems to support Franco’s contention. It also echoes the findings of Perlas et al.13 who reported a 0.4% incidence of self-resolving numbness in a series of 510 US-guided SCBs with penetration of the main cluster.
Our protocol contains some limitations. First, the clinical benefits of a 7.1-min difference in total time may seem limited. However, in high turnover surgical settings (≥ four upper extremity cases per day), the cumulative gains could translate into one additional case per day. Second, albeit supervised by an expert, novice operators carried out all TII SCBs. Since the TII SCB is predicated on needling multiple targets, we speculate that the decrease in total times would have been even more impressive had experienced operators performed the blocks. Finally, although no patient exhibited serious neurological deficits in the current and previous trials,1,4 further studies are warranted to investigate the safety profile of TII SCB.
In conclusion, US-guided TII SCB provides a quick and reliable method for brachial plexus block. Despite comparable success rates, it results in a quicker onset and shorter total anesthesia-related time than ultrasound-guided AXB.
References
Techasuk W, González AP, Bernucci F, Cupido T, Finlayson RJ, Tran DQ. A randomized comparison between double-injection and targeted intracluster-injection ultrasound-guided supraclavicular brachial plexus block. Anesth Analg 2014; 118: 1363-9.
Tran DQ, Dugani S, Correa JA, Diachenko A, Alsenosy N, Finlayson RJ. Minimum effective volume of lidocaine for ultrasound-guided supraclavicular block: a prospective, randomized, blinded controlled study. Reg Anesth Pain Med 2011; 36: 466-9.
Roy M, Nadeau MJ, Côté D, et al. Comparison of a single- or double-injection technique for ultrasound-guided supraclavicular block. Reg Anesth Pain Med 2012; 35: 55-9.
Yazer MS, Finlayson RJ, Tran DQ. A randomized comparison between infraclavicular block and targeted intracluster injection supraclavicular block. Reg Anesth Pain Med 2015; 40: 11-5.
Merchant R, Chartrand D, Dain S, et al. Guidelines to the practice of anesthesia–revised edition 2015. Can J Anesth 2015; 62: 54-79.
Konrad C, Schupfer G, Wietlisbach M, Gerber H. Learning manual skills in anesthesiology: is there a recommended number of cases for anesthetic procedures? Anesth Analg 1998; 86: 635-9.
Bernucci F, González AP, Finlayson RJ, Tran DQ. A prospective, randomized comparison between perivascular and perineural ultrasound-guided axillary brachial plexus block. Reg Anesth Pain Med 2012; 37: 473-7.
Casati A, Danelli G, Baciarello M, et al. A prospective, randomized comparison between ultrasound and nerve stimulation guidance for multiple injection axillary brachial plexus block. Anesthesiology 2007; 106: 992-6.
González AP, Bernucci F, Pham K, Correa JA, Finlayson RJ, Tran DQ. Minimum effective volume of lidocaine for double-injection ultrasound-guided axillary block. Reg Anesth Pain Med 2013; 38: 16-20.
Tran DQ, Russo G, Munoz L, Zaouter C, Finlayson RJ. A prospective, randomized comparison between ultrasound-guided supraclavicular, infraclavicular and axillary brachial plexus blocks. Reg Anesth Pain Med 2009; 34: 366-71.
Bigeleisen PE, Moayeri N, Groen GJ. Extraneural versus intraneural stimulation thresholds during ultrasound-guided supraclavicular block. Anesthesiology 2009; 110: 1235-43.
Franco CD. Connective tissues associated with peripheral nerves. Reg Anesth Pain Med 2012; 37: 363-5.
Perlas A, Lobo G, Lo N, Brull R, Chan VW, Karkhanis R. Ultrasound-guided supraclavicular block: outcome of 510 consecutive cases. Reg Anesth Pain Med 2009; 34: 171-6.
Acknowledgement
The authors are indebted to Mr. Derek Mitchell for his help with patient recruitment.
Funding
None of the authors received funding for this study.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Roderick J. Finlayson and De Q.H. Tran designed the study. All authors helped conduct the study, analyze the data, and write the manuscript. All authors have seen the original study data and reviewed the analysis of the data.
Rights and permissions
About this article
Cite this article
Arnuntasupakul, V., Leurcharusmee, P., De La Garza, D.C. et al. A randomized trial comparing axillary block versus targeted intracluster injection supraclavicular block for upper limb surgery. Can J Anesth/J Can Anesth 62, 1287–1294 (2015). https://doi.org/10.1007/s12630-015-0485-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-015-0485-x