Abstract
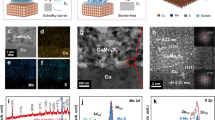
Commercial hydrogen production involves the development of efficient hydrogen evolution reaction catalysts. Herein, we adopted a friction stir processing (FSP) technique to mix immiscible metals homogenously and obtain a self-supporting copper–silver (CuAg) catalyst. The gust of Ag atoms with larger atomic sizes caused a tensile strain in the Cu matrix. Meanwhile, the chemical-potential difference induced electron transfer from Cu to Ag, and the two factors jointly led to the upshift of Cu d-band and improved the catalytic activity. Consequently, the CuAg electrode exhibited a high turnover frequency (12 times that of pure Cu), a low overpotential at high current density (superior to platinum foil), and high durability (1.57% decay over 180 h). Our work demonstrates that FSP is a powerful method for preparing self-supporting catalysts of immiscible alloys with high catalytic performance.
Similar content being viewed by others
References
Z.W. Seh, J. Kibsgaard, C.F. Dickens, I. Chorkendorff, J.K. Nørskov, and T.F. Jaramillo, Combining theory and experiment in electrocatalysis: Insights into materials design, Science, 355(2017), No. 6321, art. No. eaad4998.
J. Wang, T. Liao, Z.Z. Wei, J.T. Sun, J.J. Guo, and Z.Q. Sun, Heteroatom-doping of non-noble metal-based catalysts for electrocatalytic hydrogen evolution: An electronic structure tuning strategy, Small Methods, 5(2021), No. 4, art. No. 2000988.
Q.Q. Zhang and J.Q. Guan, Single-atom catalysts for electrocatalytic applications, Adv. Funct. Mater., 30(2020), No. 31, art. No. 2000768.
X.H. Wu, S. Zhou, Z.Y. Wang, et al., Engineering multifunctional collaborative catalytic interface enabling efficient hydrogen evolution in all pH range and seawater, Adv. Energy Mater., 9(2019), No. 34, art. No. 1901333.
H.B. Liao, C. Wei, J.X. Wang, et al., A multisite strategy for enhancing the hydrogen evolution reaction on a nano-Pd surface in alkaline media, Adv. Energy Mater., 7(2017), No. 21, art. No. 1701129.
J. Zhao, P.D. Tran, Y. Chen, J.S.C. Loo, J. Barber, and Z.J. Xu, Achieving high electrocatalytic efficiency on copper: A low-cost alternative to platinum for hydrogen generation in water, ACS Catal., 5(2015), No. 7, p. 4115.
H.N. Sun, X.M. Xu, H. Kim, W. Jung, W. Zhou, and Z.P. Shao, Electrochemical water splitting: Bridging the gaps between fundamental research and industrial applications, Energy Environ. Mater., (2023), art. No. e12441. https://doi.org/10.1002/eem2.12441.
X.D. He, X. Han, X.Y. Zhou, et al., Electronic modulation with Pt-incorporated NiFe layered double hydroxide for ultrastable overall water splitting at 1000 mA cm−2, Appl. Catal. B, 331(2023), art. No. 122683.
Z.Y. Zhou, X.R. Li, Q. Li, Y. Zhao, and H. Pang, Copper-based materials as highly active electrocatalysts for the oxygen evolution reaction, Mater. Today Chem., 11(2019), p. 169.
D. Lukács, L. Szyrwiel, and J.S. Pap, Copper containing molecular systems in electrocatalytic water oxidation—Trends and perspectives, Catalysts, 9(2019), No. 1, art. No. 83.
S. Nitopi, E. Bertheussen, S.B. Scott, et al., Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte, Chem. Rev., 119(2019), No. 12, p. 7610.
J.W. Vickers, D. Alfonso, and D.R. Kauffman, Electrochemical carbon dioxide reduction at nanostructured gold, copper, and alloy materials, Energy Technol., 5(2017), No. 6, p. 775.
J.K. Nørskov, T. Bligaard, A. Logadottir, et al., Trends in the exchange current for hydrogen evolution, J. Electrochem. Soc., 152(2005), No. 3, art. No. J23.
P.F.B.D. Martins, P.P. Lopes, E.A. Ticianelli, V.R. Stamenkovic, N.M. Markovic, and D. Strmcnik, Hydrogen evolution reaction on copper: Promoting water dissociation by tuning the surface oxophilicity, Electrochem. Commun., 100(2019), p. 30.
S.R. Zhang, L. Nguyen, J.X. Liang, et al., Catalysis on singly dispersed bimetallic sites, Nat. Commun., 6(2015), art. No. 7938.
Q. Lu, G.S. Hutchings, W.T. Yu, et al., Highly porous non-precious bimetallic electrocatalysts for efficient hydrogen evolution, Nat. Commun., 6(2015), art. No. 6567.
B. Liu, H.Q. Peng, J.Y. Cheng, et al., Nitrogen-doped graphene-encapsulated nickel–copper alloy nanoflower for highly efficient electrochemical hydrogen evolution reaction, Small, 15(2019), No. 48, art. No. 1901545.
W.C. Sheng, H.A. Gasteiger, and Y. Shao-Horn, Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes, J. Electrochem. Soc., 157(2010), No. 11, art. No. B1529.
Z. Li, J.Y. Fu, Y. Feng, C.K. Dong, H. Liu, and X.W. Du, A silver catalyst activated by stacking faults for the hydrogen evolution reaction, Nat. Catal., 2(2019), No. 12, p. 1107.
M.C. Luo and S.J. Guo, Strain-controlled electrocatalysis on multimetallic nanomaterials, Nat. Rev. Mater., 2(2017), art. No. 17059.
A. Khorshidi, J. Violet, J. Hashemi, and A.A. Peterson, How strain can break the scaling relations of catalysis, Nat. Catal., 1(2018), No. 4, p. 263.
W.J. Kang, Y. Feng, Z. Li, et al., Strain - activated copper catalyst for pH - universal hydrogen evolution reaction, Adv. Funct. Mater., 32(2022), No. 18, art. No. 2112367.
Q.L. Wu, M. Luo, J.H. Han, et al., Identifying electrocatalytic sites of the nanoporous copper–ruthenium alloy for hydrogen evolution reaction in alkaline electrolyte, ACS Energy Lett., 5(2020), No. 1, p. 192.
M.Y. Yang, L. Jiao, H.L. Dong, et al., Conversion of bimetallic MOF to Ru-doped Cu electrocatalysts for efficient hydrogen evolution in alkaline media, Sci. Bull., 66(2021), No. 3, p. 257.
J.S. Tian, Y.C. Hu, W.F. Lu, et al., Dealloying of an amorphous TiCuRu alloy results in a nanostructured electrocatalyst for hydrogen evolution reaction, Carbon Energy, (2023), art. No. e322. https://doi.org/10.1002/cey2.322.
R.F. Zhang, X.F. Kong, H.T. Wang, et al., An informatics guided classification of miscible and immiscible binary alloy systems, Sci. Rep., 7(2017), art. No. 9577.
S.L. Guo, S.P. Liu, J.C. Liu, Z.S. Gao, D.F. Li, and Z.G. Liu, Investigation on strength, ductility and electrical conductivity of Cu–4Ag alloy prepared by cryorolling and subsequent annealing process, J. Mater. Eng. Perform., 28(2019), No. 11, p. 6809.
C.C. Zhao, R.M. Niu, Y. Xin, et al., Improvement of properties in Cu-Ag composites by doping induced microstructural refinement, Mater. Sci. Eng. A, 799(2021), art. No. 140091.
Y.Z. Tian, S.D. Wu, Z.F. Zhang, R.B. Figueiredo, N. Gao, and T.G. Langdon, Comparison of microstructures and mechanical properties of a Cu–Ag alloy processed using different severe plastic deformation modes, Mater. Sci. Eng. A, 528(2011), No. 13–14, p. 4331.
H. Li, C.Y. Guo, and C.L. Xu, A highly sensitive non-enzymatic glucose sensor based on bimetallic Cu–Ag superstructures, Biosens. Bioelectron., 63(2015), p. 339.
H.M. Sun, Z.H. Yan, F.M. Liu, W.C. Xu, F.Y. Cheng, and J. Chen, Self-supported transition-metal-based electrocatalysts for hydrogen and oxygen evolution, Adv. Mater., 32(2020), No. 3, art. No. 1806326.
S. Sultan, J.N. Tiwari, A.N. Singh, et al., Single atoms and clusters based nanomaterials for hydrogen evolution, oxygen evolution reactions, and full water splitting, Adv. Energy Mater., 9(2019), No. 22, art. No. 1900624.
J. Li, J. Zhang, J.K. Shen, et al., Self-supported electrocatalysts for the hydrogen evolution reaction, Mater. Chem. Front., 7(2023), No. 4, p. 567.
C.H. Zhang, Z. Xu, N.N. Han, et al., Superaerophilic/superaerophobic cooperative electrode for efficient hydrogen evolution reaction via enhanced mass transfer, Sci. Adv., 9(2023), No. 3, art. No. eadd6978.
M. Miličić, P. Gladović, R. Bojanić, T. Savković, and N. Stojić, Friction stir welding (FSW) process of copper alloys, Metalurgija, 55(2016), No. 1, p. 107.
L. Cui, C. Zhang, Y.C. Liu, X.G. Liu, D.P. Wang, and H.J. Li, Recent progress in friction stir welding tools used for steels, J. Iron Steel Res. Int., 25(2018), No. 5, p. 477.
Y. Huang, J.L. Du, and Z.M. Wang, Progress in research on the alloying of binary immiscible metals, Acta Metall. Sin., 56(2020), No. 6, p. 801.
P. Xue, Z.Y. Huang, B.B. Wang, et al., Intrinsic high cycle fatigue behavior of ultrafine grained pure Cu with stable structure, Sci. China Mater., 59(2016), No. 7, p. 531.
M. Komarasamy, R.S. Mishra, S. Mukherjee, and M.L. Young, Friction stir-processed thermally stable immiscible nanostructured alloys, JOM, 67(2015), No. 12, p. 2820.
M. Komarasamy, R. Tharp, S. Sinha, S. Thapliyal, and R. Mishra, Achieving forced mixing in Cu-based immiscible alloys via friction stir processing, [in] Y. Hovanski, R. Mishra, Y. Sato, P. Upadhyay, and D. Yan, eds., Friction Stir Welding and Processing X, Springer, Cham, 2019, p. 199.
Y. Feng, Z. Li, S. Kang, et al., Mechanically processing copper plate into active catalyst for electrochemical hydrogen production, Acta Mater., 237(2022), art. No. 118164.
T.T. Yang, C.Q. Cheng, L.Y. Xiao, et al., A descriptor of IB alloy catalysts for hydrogen evolution reaction, SmartMat, (2023), art. No. e1204. https://doi.org/10.1002/smm2.1204.
N. Behrooz, A. Ghaffarinejad, and N. Sadeghi, Ag/Cu nano alloy as an electrocatalyst for hydrogen production, J. Electroanal. Chem., 782(2016), p. 1.
E. Rafiee, M. Farzam, M.A. Golozar, and A. Ashrafi, An investigation on dislocation density in cold-rolled copper using electrochemical impedance spectroscopy, ISRN Corros., 2013(2013), art. No. 921825.
M.H. Xie, S.Q. Ai, J. Yang, Y.D. Yang, Y.H. Chen, and Y. Jin, In-situ generation of oxide nanowire arrays from AgCuZn alloy sulfide with enhanced electrochemical oxygen-evolving performance, ACS Appl. Mater. Interfaces, 7(2015), No. 31, p. 17112.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 51871160 and 52101266) and the Natural Science Foundation of Hefei Grant (No. 2022046).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Information
Rights and permissions
About this article
Cite this article
Hu, X., Liu, Z., Feng, Y. et al. Mechanically mixing copper and silver into self-supporting electrocatalyst for hydrogen evolution. Int J Miner Metall Mater 30, 1906–1913 (2023). https://doi.org/10.1007/s12613-023-2695-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-023-2695-5