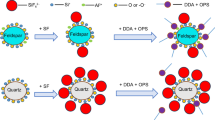
Abstract
The separation of andalusite and quartz was investigated in the sodium oleate flotation system, and its mechanism was studied by solution chemical calculation, zeta-potential tests, Fourier transform infrared spectroscopic (FTIR), and X-ray photoelectron spectroscopic (XPS). The flotation tests results show that FeCl3·6H2O has a strong activation effect on andalusite and quartz and citric acid has a strong inhibitory effect on activated quartz, thus increasing the floatability difference between quartz and andalusite when the pulp pH is approximately 8. The FTIR, Zeta potential, and XPS analyses combined with the chemical calculation of flotation reagent solutions demonstrate that Fe forms hydroxide precipitates on the surface of andalusite and quartz and that oleate anions and metal ions adsorb onto the surface of the minerals. The elements Al and Fe can be chemically reacted. The anions in citric acid have different degrees of dissolution of Fe on the andalusite and quartz surfaces, thereby selectively eliminating the activation of the elemental Fe on andalusite and quartz and increasing the floatability of andalusite, leading to a better separation effect between andalusite and quartz.
Similar content being viewed by others
References
J.F. Shackelford and R.H. Doremus, Ceramic and Glass Materials: Structure, Properties and Processing, Springer, New York, 2008, p. 41.
A. Tomba, M.A. Camerucci, G. Urretavizcaya, A.L. Cavalieri, M.A. Sainz, and A. Caballero, Elongated mullite crystals obtained from high temperature transformation of sillimanite, Ceram. Int., 25(1999), No. 3, p. 245.
C.Y. Sun and W.Z. Yin, Principle of Silicate Mineral Flotation, Science Press, Beijing, 2001, p. 447.
B.Y. Lin, X.Y. Zhang, Y.F. Ding, and C.J. Guo, Current situation and prospect of China's “three stones”, [in] Proceedings of the Symposium on Comprehensive, Coordinated and Sustainable Development of China's Refractory Industry, Xiamen, 2005, p. 147.
J. Lu, Z.J. Ren, H.M. Gao, Z.C. Yi, Z.J. Chen, Y. Huang, and J.Y. Wen, Experimental research on preconcentration of an andalusite ore in Xinjiang, Non-Metallic Mines, 39(2016), No. 5, p. 69.
B.Y. Lin, Kyanite Andalusite Sillimanite, Metallurgical Industry Press, Beijing, 1998, p. 22.
X.Y. Song, Research on Mineral Processing Technology of Xixia and Alusite Ore in Henan Province [Dissertation], China University of Geosciences, Beijing, 2007, p. 13.
F. Ren, Y.X. Han, W.Z. Yin, Z.H. Wang, Z.T. Yuan, and Z.X. Wang, Solution chemical analysis of sodium oleate flotation tourmaline, Non-Ferrous Min. Metall., 21(2005), No. 7, p. 158.
X.M. Luo, Study on the Interaction of Minerals in Carbonate Iron Ore Flotation System [Dissertation], Northeastern University, Shenyang, 2014, p. 129.
J.X. Jin, Study on Flotation Behavior and Mechanism of Homogeneous Polymorphic Minerals of Andalusite [Dissertation], Wuhan University of Technology, Wuhan, 2016, p. 3.
S.F. Weng and Y.Z. Xu, Fourier Transform Infrared Spectroscopy, Chemical Industry Press, Beijing, 2016, p. 297.
J. Yao, D. Li, W.Z. Yin, and H.L. Han, Dispersion mechanism of citric acid in flotation system containing carbonate, J. Northeastern Univ. Nat. Sci., 38(2017), No. 5, p. 720.
Y.J. Peng, B. Wang, and A. Gerson, The effect of electrochemical potential on the activation of pyrite by copper and lead ions during grinding, Int. J. Miner. Process., 102–103(2012), p. 141.
L. Wang, W. Sun, Y.H. Hu, and L.H. Xu, Adsorption mechanism of mixed anionic/cationic collectors in Muscovite-Quartz flotation system, Miner. Eng., 64(2014), p. 44.
J.B. Metson, Charge compensation and binding energy referencing in XPS analysis, Surf. Interface Anal., 27(1999), No. 12, p. 1069.
Acknowledgement
This work was financially supported by the State Key Laboratory of Mineral Processing of BGRIMM Technology Group, China (No. BGRIMM-KJSKL-2017-11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, Yp., Sun, Cy., Yin, Wz. et al. Selective flotation separation of andalusite and quartz and its mechanism. Int J Miner Metall Mater 26, 1059–1068 (2019). https://doi.org/10.1007/s12613-019-1842-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-019-1842-5