Abstract
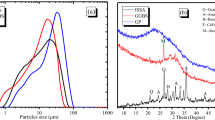
The effects of sericite particle size, rotation speed, and leaching temperature on sericite dissolution and copper extraction in a chalcopyrite bioleaching system were examined. Finer particles, appropriate temperature and rotation speed for Acidithiobacillus ferrooxidans resulted in a higher Al3+ dissolution concentration. The Al3+ dissolution concentration reached its highest concentration of 38.66 mg/L after 48-d leaching when the sericite particle size, temperature, and rotation speed were −43 μm, 30°C, and 160 r/min, respectively. Meanwhile, the sericite particle size, rotation speed, and temperature can affect copper extraction. The copper extraction rate is higher when the sericite particle size is finer. An appropriately high temperature is favorable for copper leaching. The dissolution of sericite fitted the shrinking core model, 1–(2/3)α–(1–α)2/3 = k 1 t, which indicates that internal diffusion is the decision step controlling the overall reaction rate in the leaching process. Scanning electron microscopy analysis showed small precipitates covered on the surface of sericite after leaching, which increased the diffusion resistance of the leaching solution and dissolved ions.
Similar content being viewed by others
References
H.R. Watling, The bioleaching of sulphide minerals with emphasis on copper sulphides: a review, Hydrometallurgy, 84(2006), No. 1-2, p. 81.
Y.G. Guo, P. Huang, W.G. Zhang, X.W. Yuan, F.X. Fan, H.L. Wang, J.S. Liu, and Z.H. Wang, Leaching of heavy metals from Dexing copper mine tailings pond, Trans. Nonferrous Met. Soc. China, 23(2013), No. 10, p. 3068.
X.L. Mo, H. Lin, K.B. Fu, Y.B. Dong, and C.Y. Xu, Effect of sericite on bioleaching of chalcopyrite, Chin. J. Nonferrous Met., 22(2012), No. 5, p. 1475.
M. Dopson, L. Lövgren, and B. Dan, Silicate mineral dissolution in the presence of acidophilic microorganisms: Implications for heap bioleaching, Hydrometallurgy, 96(2009), No. 4, p. 288.
V. Ochoaherrera, G. León, Q. Banihani, J.A. Field, and R. Sierraalvarez, Toxicity of copper(II) ions to microorganisms in biological wastewater treatment systems, Sci. Total Environ., 412–413(2011), No. 13, p. 380.
J. Fischer, A. Quentmeier, S. Gansel, V. Sabados, and C.G. Friedrich, Inducible aluminium resistance of Acidiphilium cryptum and aluminium tolerance of other acidophilic bacteria, Arch. Microbiol., 178(2002), No. 6, p. 554.
K.R. Blight and D.E. Ralph, Aluminium sulphate and potassium nitrate effects on batch culture of iron oxidising bacteria, Hydrometallurgy, 92(2008), No. 3-4, p. 130.
M. Suwalsky, B. Norris, F. Villena, F. Cuevas, P. Sotomayor, and P. Zatta, Aluminum fluoride affects the structure and functions of cell membranes, Food Chem. Toxicol., 42(2004), No. 6, p. 925.
Y.B. Dong, H. Lin, H. Wang, X.L. Mo, K.B. Fu, and H.W. Wen, Effects of ultraviolet mutation on bioleaching of low-grade copper tailings, Miner. Eng., 24(2011), No. 8, p. 870.
D. Bingöl, M. Canbazoğlu, and S. Aydoğan, Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching, Hydrometallurgy, 76(2005), No. 1-2, p. 55.
David R. Ely, R. Edwin Garcíab, and M. Thommes, Ostwald–Freundlich diffusion-limited dissolution kinetics of nanoparticles, Powder Technol., 257(2014), p. 120.
A. Sanna, A. Lacinska, M. Styles, and M.M. Maroto-Valer, Silicate rock dissolution by ammonium bisulphate for pH swing mineral CO2 sequestration, Fuel Process. Technol., 120(2010), No. 4, p. 128.
A.A. Baba and F.A. Adekola, A study of dissolution kinetics of a Nigerian galena ore in hydrochloric acid, J. Saudi Chem. Soc., 16(2012), No. 4, p. 377.
M. Gleisner, R.B.H. Jr, and P.C.F. Kockuma, Pyrite oxidation by Acidithiobacillus ferrooxidans at various concentrations of dissolved oxygen, Chem. Geol., 225(2006), No. 1-2, p. 16.
Z.Y. Ding, Z.L. Yin, H.P. Hu, and Q.Y. Chen, Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution, Hydrometallurgy, 104(2010), No. 2, p. 201.
K.C. Liddell, Shrinking core models in hydrometallurgy: What students are not being told about the pseudo-steady approximation, Hydrometallurgy, 79(2005), No. 1-2, p. 62.
R. Salmimies, M. Mannila, J. Kallas, and A. Häkkinen, Acidic dissolution of hematite: kinetic and thermodynamic investigations with oxalic acid, Int. J. Miner. Process., 110-111(2012), p. 121.
T.J. Hu, G.M. Zeng, and X.Z. Yuan, Leaching kinetics of silver extracted by thiourea from residue in hydrometallurgy of zinc, Chin. J. Nonferrous Met., 11(2001), No. 5, p. 933.
S.H. Ju, M.T. Tang, S.H. Yang, and Y. Li, Dissolution kinetics of smithsonite ore in ammonium chloride solution, Hydrometallurgy, 80(2005), No. 1, p. 67.
V. Safari, G. Arzpeyma, F. Rashchi, and N. Mostoufi, A shrinking particle-shrinking core model for leaching of a zinc ore containing silica, Int. J. Miner. Process., 93(2009), No. 1, p. 79.
A. Amiri, G.D. Ingram, A.V. Bekker, I. Livk, and N.E. Maynard, A multi-stage, multi-reaction shrinking core model for self-inhibiting gas–solid reactions, Adv. Powder Technol., 24(2013), No. 4, p. 728.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51204011) and the Science and Technology Project for the Guidance Teacher of Beijing Excellent Doctoral Dissertation (No. 20121000803).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, Yb., Li, H., Lin, H. et al. Dissolution characteristics of sericite in chalcopyrite bioleaching and its effect on copper extraction. Int J Miner Metall Mater 24, 369–376 (2017). https://doi.org/10.1007/s12613-017-1416-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-017-1416-3