Abstract
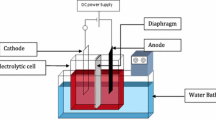
The effects of Br− and I− concentrations on the cell voltage, anodic polarization, current efficiency (CE), and energy consumption (EC) of zinc electrodeposition from ammoniacal ammonium chloride solutions were investigated. The surface morphology of zinc deposits was also examined. Scanning electron microscopy (SEM) and X-ray diffraction (XRD) were used to characterize the morphology of zinc deposits and the phase of anodic sediments produced during electrolysis. The results clearly showed that the CE slightly increased from approximately 95.12% in the absence of I− and Br− to 97.08% in the presence of 10 g·L−1 Br−; in contrast, the CE significantly decreased to less than 83% in the presence of 10 g·L−1 I−. The addition of Br− and I− positively affected the EC, which decreased from 2514 kW·h·t−1 to approximately 2300 kW·h·t−1. The results of anodic polarization measurements showed that the voltage drops were 130 and 510 mV when the concentrations of Br− and I− were 10 g·L−1 at a current density of 400 A·m−2, respectively. SEM images showed that the addition of Br− and I− caused different crystal growth mechanisms, which resulted in the production of compact and smooth zinc deposits. The anodic reactions of I− were also studied.
Similar content being viewed by others
References
T. Suetens, B. Klaasen, K. Van Acker, and B. Blanpain, Comparison of electric arc furnace dust treatment technologies using exergy efficiency, J. Clean. Prod., 65(2014), p. 152.
M. Liebman, The current status of electric arc furnace dust recycling in North America, [in] Recycling of Metals and Engineered Materials, Hoboken, 2000, p. 237.
J. Rütten, C. Frias, G. Diaz, D. Martin, and F. Sanchez, Processing EAF dust through Waelz Kiln and ZINCEXTM solvent extraction: the optimum solution, [in] European Metallurgical Conference, Dusseldorf, 2011, p. 1673.
A.D. Zunkel, Recovering zinc and lead from electric arc furnace dust: a technology status report, [in] Recycling of Metals and Engineered Materials, Hoboken, 2000, p. 227.
S.S. Chabot and S.E. James, Treatment of secondary zinc oxides for use in an electrolytic zinc plant, [in] Recycling of Metals and Engineered Materials, Hoboken, 2000, p. 739.
M. Olper and M. Maccagni, Electrolytic zinc production from crude zinc oxides with the Ezinex® process, [in] Recycling of Metals and Engineered Materials, Hoboken, 2000, p. 269.
M.T. Tang and S.H. Yang, Electrowinning zinc in the system of Zn(?)-NH3-NH4Cl-H2O and mechanism of anodic reaction, J. Cent. South Univ. Technol., 30(1999), No. 2, p. 153.
R.X. Wang, M.T. Tang, W. Liu, S.H. Yang, and W.H. Zhang, Leaching of low-grade zinc oxide ore in NH3-NH4Cl-H2O system for production of electrolytic zinc, Chin. J. Process Eng., 8(2008), Suppl. 1, p. 219.
B.P. Zhang, M.T. Tang, and S.H. Yang, Treating zinc oxide ores using ammonia-ammonium chloride to produce electrolysis zinc, J. Cent. South. Univ. Technol., 34(2003), No. 6, p. 619.
S.H. Ju, M.T. Tang, S.H. Yang, and Y.N. Li, Dissolution kinetics of smithsonite ore in ammonium chloride solution, Hydrometallurgy, 80(2005), No. 1-2, p. 67.
W.C. Korbach, N.J. William, Howell, H. McMullen, E. Warren, and Brunswick, High Performance Electrodeposited Chromium Layers, American Patent, Appl. 4828656, 1987.
W.C. He, G.Q. Liu, W. Cui, and Y. Tang, Effects of potassium iodide additive on direct electrosynthesis of potassium ferrate, Inorg. Chem. Ind., 43(2011), No. 2, p. 20.
W.H. Yang, D.Q. Bi, Y. Zhou, H.H. Wang, and X.R. Lan, Selection of addition in the process of electrochemical synthesizing of K2FeO4, Electrochemistry, 13(2007), No. 4, p. 445.
A.M. Alfantazi and A. Shakshouki, The effects of chloride ions on the electrowinning of nickel from sulfate electrolytes, J. Electrochem. Soc., 149(2002), No. 10, p. C506.
M. Olper and M. Maccagni, From C.Z.O. to zinc cathode without any pre-treatment, [in] Proceedings of the EZINEX Process, Lead and Zinc 2008, Durban, 2008, p. 85.
Z.M. Xia, S.H. Yang, M.T. Tang, T.Z. Yang, Z.H. Liu, C.B. Tang, J. He, and X.L. Deng, Cycle leaching of low grade zinc oxide ores in MACA system for preparing zinc, Chin. J. Nonferrous Met., 23(2013), No. 12, p. 3455.
A.J. Bard, R. Parsons, and J. Jordan, Standard Potentials in Aqueous Solution, CRC Press, New York and Bessel, 1985, p. 221.
S.H. Yang, Theory and Application Studies on Preparing High Purity Zinc in the System of Zn(II)-NH 3-NH4Cl-H2 O [Dissertation], Central South University, Changsha, 2003, p. 32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xia, Zm., Yang, Sh., Duan, Lh. et al. Effects of Br− and I− concentrations on Zn electrodeposition from ammoniacal electrolytes. Int J Miner Metall Mater 22, 682–687 (2015). https://doi.org/10.1007/s12613-015-1122-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12613-015-1122-y