Abstract
Purpose of Review
Advances in radiotherapy technology have allowed for highly conformal, high-dose stereotactic body radiotherapy (SBRT) to be delivered in just 1–5 fractions, which opened a plethora of new treatment options for both early-stage and metastatic breast cancer (BC). This review discusses recent studies and ongoing trials for SBRT in BC.
Recent Findings
In early-stage BC, adjuvant accelerated and ultra-hypofractionated radiation now have high-quality evidence showing similar outcomes compared to moderately hypofractionated radiation. Studies are also investigating the role of neoadjuvant and definitive SBRT for early-stage BC. In oligometastatic disease, randomized evidence shows improved outcomes when SBRT is combined with systemic therapy, and multiple BC-specific trials are attempting to elucidate optimal patient selection and treatment regimens. While SBRT for oligoprogression has shown mixed findings, the synergy of immunologic changes and improving systemic therapies is likely to enhance treatment efficacy in the future.
Summary
Outcomes of recent SBRT trials show favorable results across a multitude of BC stages. By highlighting existing and ongoing trials, we hope to improve the availability of SBRT for BC patients.

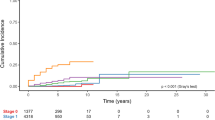
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
American Cancer Society. American Cancer Society: cancer facts and figures 2021. Atlanta, Ga Am. Cancer Soc; 2021
American Cancer Society Breast cancer facts & figures 2019–2020.
Meattini I, Marrazzo L, Saieva C, et al. Accelerated partial-breast irradiation compared with whole-breast irradiation for early breast cancer: long-term results of the randomized phase III APBI-IMRT-florence trial. J Clin Oncol. 2020;38:4175–4183. This phase III trial compared adjuvant WBI to APBI with five-fraction IMRT for early-stage BC. Ten-year ipsilateral breast recurrence, OS, and BC-specific survival were not statistically different. The APBI arm showed significantly less acute and late toxicity with improved cosmetic outcomes.
Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. This phase III equivalency trial randomized early-stage BC patients to WBI versus APBI with BID schedule. Equivalency for ten-year ipsilateral breast tumor recurrence was not met although the absolute difference at ten years was 0.7%.
Agrawal V, Hissourou M, Fenton-Kerimian MB, et al. Prone accelerated partial breast irradiation comparing five versus three fractions for early stage breast cancer: a multi-institutional prospective randomized trial. Int J Radiat Oncol. 2020;108:S153–4.
Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of fast: a randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38:3261–72.
Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. This phase III trial randomized patients with early-stage BC to hypofractionated 15-fraction WBI versus five-fraction WBI (26 Gy or 27 Gy). The 26 Gy in five fractions regimen was determined to be non-inferior to conventional WBI with no difference in tumor control or normal tissue effects up to 5 years.
Whelan TJ, Pignol J-P, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20.
Haviland JS, Owen JR, Dewar JA, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–94.
Formenti SC, Hsu H, Fenton-Kerimian M, Roses D, Guth A, Jozsef G, Goldberg JD, Dewyngaert JK. Prone accelerated partial breast irradiation after breast-conserving surgery: five-year results of 100 patients. Int J Radiat Oncol Biol Phys. 2012;84:606–11.
Whelan TJ, Julian JA, Berrang TS, et al (2019) External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. This phase III non-inferiority trial randomized early-stage BC patients to WBI versus APBI (38.5 Gy in 10 fractions twice a day). There was no difference in ipsilateral breast tumor recurrence at 8 years. Less acute toxicity was seen in the APBI arm. However, adverse cosmesis and late toxicity were more common with APBI.
Van Der Leij F, Elkhuizen PH, Janssen TM, Poortmans P, Van Der Sangen M, Scholten AN, Van Vliet-Vroegindeweij C, Boersma LJ. Target volume delineation in external beam partial breast irradiation: less inter-observer variation with preoperative-compared to postoperative delineation. Radiother Oncol. 2014;110:467–70.
Guidolin K, Yaremko B, Lynn K, et al. Stereotactic image-guided neoadjuvant ablative single-dose radiation, then lumpectomy, for early breast cancer: the signal prospective single-arm trial of single-dose radiation therapy. Curr Oncol. 2019;26:334–40.
(2021) Stereotactic body radiation therapy for breast cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03585621. Accessed 29 Dec 22
Horton JK, Blitzblau RC, Yoo S, et al. Preoperative single-fraction partial breast radiation therapy: a novel phase 1, dose-escalation protocol with radiation response biomarkers. Int J Radiat Oncol. 2015;92:846–55.
(2021) Feasibility study of stereotactic body radiotherapy for early breast cancer (ARTEMIS). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02065960. Accessed 29 Dec 22
(2021) Preoperative stereotactic ablative body radiotherapy (SABR) for early-stage breast cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03137693. Accessed 29 Dec 22
(2021) Preoperative single-fraction radiotherapy in early stage breast cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02482376. Accessed 29 Dec 22
(2021) Study of stereotactic radiotherapy for breast cancer. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03043794. Accessed 29 Dec 22
(2021) Single dose ablative radiation treatment for early-stage breast cancer (ABLATIVE). ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02316561. Accessed 29 Dec 22
Hellman S, Weichselbaum RR. Oligometastasis editorial. J Clin Oncol. 1995;13:8–10.
Gianni L, Romieu GH, Lichinitser M, et al. AVEREL: a randomized phase III trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for her2-positive locally recurrent/metastatic breast cancer. In: J. Clin. Oncol. 2013;1719–1725
Albain KS, Nag SM, Calderillo-Ruiz G, et al. Gemcitabine plus paclitaxel versus paclitaxel monotherapy in patients with metastatic breast cancer and prior anthracycline treatment. J Clin Oncol. 2008;26:3950–7.
Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31:1157–63.
Tawfik H, Rostom Y, Elghazaly H. All-oral combination of vinorelbine and capecitabine as first-line treatment in HER2/Neu-negative metastatic breast cancer. Cancer Chemother Pharmacol. 2013;71:913–9.
Bergh J, Bondarenko IM, Lichinitser MR, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–9.
Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393:2051–8.
Palma DA, Olson R, Harrow S, et al (2020) Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol 38:2830–2838. This phase II trial randomized patients with a controlled primary malignancy and 1–5 metastasic lesions to standard-of-care treatments versus SABR. The OS and PFS benefit with SABR was durable at 5 years with no detrimental impact on quality of life.
Gomez DR, Blumenschein GR Jr, Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672–82.
Gomez DR, Tang C, Zhang J, Blumenschein Jr GR, Hernandez M. Original report abstract local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non – small-cell lung cancer: long-term results of a multi institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37(18):1558-1565.
Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative Radiotherapy for Limited Metastatic Non–Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018;4(1):e173501–e173501.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6(5):650–9.
Ost P, Reynders D, Decaestecker K, Lumen N, Bruycker D, Delrue L, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446–53.
Viani GA, Gouveia AG, Louie AV., Korzeniowski M, Pavoni JF, Hamamura AC, Moraes FY (2021) Stereotactic body radiotherapy to treat breast cancer oligometastases: a systematic review with meta-analysis: SBRT for breast cancer oligometastases. Radiother Oncol 164:245–250. This meta-analysis evaluated the effectiveness of SABR for oligometastatic disease in BC. Ten studies met inclusion criteria and showed high rates of LC and improved OS for those treated with SABR on prospective trials or with bone-only metastases. Rates of grade 2 or 3 toxicity were low.
Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, Okunieff P. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer. 2008;112:650–8.
Wong AC, Watson SP, Pitroda SP, et al. Clinical and molecular markers of long-term survival after oligometastasis-directed stereotactic body radiotherapy (SBRT). Cancer. 2016;122:2242–50.
Olson R, Jiang W, Liu M, Bergman A, Schellenberg D, Mou B, et al. Population based phase II trial of stereotactic ablative radiotherapy (SABR) for up to 5 oligometastases: preliminary results of the SABR-5 trial. Int J Radiat Oncol Biol Phys. 2021;111(3):S4–S4.
Vanpouille-Box C, Alard A, Aryankalayil MJ, Sarfraz Y, Diamond JM, Schneider RJ, Inghirami G, Coleman CN, Formenti SC, Demaria S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618.
Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti–CTLA-4 antibody. Clin Cancer Res. 2009;15:5379–88.
Oba T, Long MD, Keler T, Marsh HC, Minderman H, Abrams SI, Liu S, Ito F. Overcoming primary and acquired resistance to anti-PD-L1 therapy by induction and activation of tumor-residing cDC1s. Nat Commun. 2020;11:5415.
Muraro E, Furlan C, Avanzo M, et al (2017) Local high-dose radiotherapy induces systemic immunomodulating effects of potential therapeutic relevance in oligometastatic breast cancer. Front Immunol. https://doi.org/10.3389/fimmu.2017.01476
Chmura S, Winter KA, Robinson C, et al (2021) Evaluation of safety of stereotactic body radiotherapy for the treatment of patients with multiple metastases: findings from the NRG-BR001 phase 1 trial. In: JAMA Oncol. 2021;845–52. This phase I study showed SBRT was safe with low toxicity rates for patients with multiple oligometastatic lesions in breast, prostate, or non-small cell lung cancer.
David S. Stereotactic ablative body radiotherapy (SABR) for bone only oligometastatic breast cancer: A prospective clinical trial. Breast. 2020;49:55–62. https://doi.org/10.1016/j.breast.2019.10.016.
Trovo M, Furlan C, Polesel J, Fiorica F, Arcangeli S, Giaj-levra N, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol. 2018;126(1):177–80. https://doi.org/10.1016/j.radonc.2017.08.032.
Scorsetti M, Franceschini D, De Rose F, Comito T, Villa E, Iftode C, et al. Stereotactic body radiation therapy: A promising chance for oligometastatic breast cancer. Breast. 2016;26:11–7.
Tsai CJ, Yang JT, Guttmann DM, et al. Consolidative use of radiotherapy to block (CURB) oligoprogression − interim analysis of the first randomized study of stereotactic body radiotherapy in patients with oligoprogressive metastatic cancers of the lung and breast. Int J Radiat Oncol. 2021;111:1325–1326. This phase II trial randomized breast and lung cancer patients with oligoprogressive disease to SBRT versus standard of care. Planned interim analyses show improved median PFS with SBRT -- however this difference was driven by the patients with lung cancer and no difference was seen in the breast cohort. Full accrural and final analyses pending.
Hanrahan EO, Broglio KR, Buzdar AU, Theriault RL, Valero V, Crisiofanilli M, et al. Combined-modality treatment for isolated recurrences of breast carcinoma: Update on 30 years of experience at the University of Texas M. D. Anderson Cancer Center and assessment of prognostic factors. Cancer. 2005;104(6):1158–71.
Milano MT, Katz AW, Zhang H, Huggins CF, Aujla KS, Okunieff P. Oligometastatic breast cancer treated with hypofractionated stereotactic radiotherapy: some patients survive longer than a decade. Radiother Oncol. 2019;131:45–51.
Siva S, Bressel M, Mai T, et al (2021) Single-fraction vs multifraction stereotactic ablative body radiotherapy for pulmonary oligometastases (SAFRON II): the Trans Tasman Radiation Oncology Group 13.01 phase 2 randomized clinical trial. JAMA Oncol 7:1476. This phase II trial randomized patients with pulmonary oligometastases to single-fraction (28 Gy) versus multifraction (48 Gy in four fractions) SBRT. No significant differences were found for LC, OS, or toxicity.
Patel PH, Palma D, McDonald F, Tree AC. The dandelion dilemma revisited for oligoprogression: treat the whole lawn or weed selectively? Clin Oncol. 2019;31:824–33.
Al-Halabi H, Sayegh K, Digamurthy SR, Niemierko A, Piotrowska Z, Willers H, Sequist LV. Pattern of failure analysis in metastatic EGFR-mutant lung cancer treated with tyrosine kinase inhibitors to identify candidates for consolidation stereotactic body radiation therapy. J Thorac Oncol. 2015;10:1601–7.
Kelly P, Ma Z, Baidas S, Moroose R, Shah N, Dagan R, Mamounas E, Rineer J (2017) Patterns of progression in metastatic estrogen receptor positive breast cancer: an argument for local therapy. Int J Breast Cancer. https://doi.org/10.1155/2017/1367159
Weiss J, Kavanagh B, Deal A, et al. Phase II study of stereotactic radiosurgery for the treatment of patients with oligoprogression on erlotinib. Cancer Treat Res Commun. 2019;19:100126.
Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:346–51.
Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–14.
Alomran R, White M, Bruce M, Bressel M, Roache S, Karroum L, Hanna GG, Siva S, Goel S, David S. Stereotactic radiotherapy for oligoprogressive ER-positive breast cancer (AVATAR). BMC Cancer. 2021;21:303.
Kinchen CL, Taylor TN, Johnstone CA, Robbins JR. Stereotactic body radiation therapy for palliative treatment of bone metastases: practice patterns and survival outcomes. J Clin Oncol. 2017;35:242–242.
Sahgal A, Myrehaug SD, Siva S, et al (2021) Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22:1023–1033. This phase II/III trial randomized patients with painful spinal metastases to SBRT (24 Gy in 2 fractions) compared to conventional 20 Gy in 5 fractions. The SBRT arm showed higher rates of complete response to pain at 3 months.
Nguyen QN, Chun SG, Chow E, et al (2019) Single-fraction stereotactic vs conventional multifraction radiotherapy for pain relief in patients with predominantly nonspine bone metastases: a randomized phase 2 trial. JAMA Oncol. 2019;5:872–878. This phase II trial randomized patients with painful bone metastases to single-fraction SBRT (12 or 16 Gy) to conventional 30 Gy in 10 fractions. The SBRT arm had more pain responders at 2 weeks which persisted to 9 months, with no difference in toxicity or quality of life. SBRT also showed higher rates of LC.
Ahmed KA, Caudell JJ, El-Haddad G, et al (2016) Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. In: Int. J. Radiat. Oncol. Biol. Phys. 2016;1399–1404
Ahmed KA, Scott JG, Arrington JA, et al. Radiosensitivity of lung metastases by primary histology and implications for stereotactic body radiation therapy using the genomically adjusted radiation dose. J Thorac Oncol. 2018;13:1121–7.
Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–6.
Major PP, Cook RJ, Lipton A, Smith MR, Terpos E, Coleman RE. Natural history of malignant bone disease in breast cancer and the use of cumulative mean functions to measure skeletal morbidity. BMC Cancer. 2009;9:272.
Ryu S, Deshmukh S, Timmerman RD, et al. Radiosurgery compared to external beam radiotherapy for localized spine metastasis: phase III results of NRG oncology/RTOG 0631. Int J Radiat Oncol. 2019;105:S2–3.
Lawrence MS, Stojanov P, Polak P, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8.
Adams S, Loi S, Toppmeyer D, et al. Phase 2 study of pembrolizumab as first-line therapy for PD-L1–positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort B. J Clin Oncol. 2017;35:1088–1088.
Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21.
Emens LA, Esteva FJ, Beresford M, et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020;21:1283–95.
Ye JC, Formenti SC. Integration of radiation and immunotherapy in breast cancer - treatment implications. The Breast. 2018;38:66–74.
Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology. 2014;3:e28518.
Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC. An abscopal response to radiation and ipilimumab in a patient with metastatic non–small cell lung cancer. Cancer Immunol Res. 2013;1:365–72.
Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–4.
Ho AY, Barker CA, Arnold BB, et al (2020) A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple‐negative breast cancer. Cancer. 2020;126:850–860. This phase II trial enrolled patients with metastatic TNBC and administered pembrolizumab and SBRT (30 Gy in 5 fractions). Overall response rate at 13 weeks was 17.6% with no grade 4 or higher toxicity.
Vanpouille-Box C, Formenti SC, Demaria S. Toward precision radiotherapy for use with immune checkpoint blockers. Clin Cancer Res. 2018;24:259–65.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
April Vassantachart, Derrick Lock, Hye Ri Han, and Jason C. Ye declare that they have no conflict of interest. None of the authors have any relevant financial or non-financial relationships or conflict of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Clinical Trials
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vassantachart, A.K., Lock, D., Han, H.R. et al. Stereotactic Body Radiation in Breast Cancer — Definitive, Oligometastatic, and Beyond. Curr Breast Cancer Rep 14, 53–64 (2022). https://doi.org/10.1007/s12609-022-00447-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12609-022-00447-1