Abstract
Metabolic syndrome (MetS) is a global epidemic complex and will cause serious metabolic comorbidities without treatment. A prevention strategy for MetS development has been proposed to modulate gut microbiota by probiotic administration to improve intestinal dysbiosis and benefit the host. Lacticaseibacillus casei LC2W has exhibited positive effects in preventing colitis and anti-hypertension in vivo. However, the effect of L. casei LC2W on subjects at high risk of MetS is unknown. Here, a randomized, double-blinded, placebo-controlled study was conducted on 60 subjects with high risk of MetS, and the hypoglycemic and hypolipidemic activity and possible pathways of L. casei LC2W were inferred from the correlation analysis with gut microbiome composition, function, and clinical phenotypic indicators. The results showed that oral administration of L. casei LC2W could exert significant benefits on weight control, glucose and lipid metabolism, inflammatory and oxidative stress parameters, and SCFA production, as well as modulate the composition of gut microbiota. The relative abundance of Lacticaseibacillus, Bifidobacterium, Dorea, and Blautia was enriched, and their interaction with other gut microbes was strengthened by oral administration of L. casei LC2W, which was beneficial in ameliorating gut inflammation, promoting glucose and lipids degradation pathways, thus alleviated MetS. The present study confirmed the prevention effects of L. casei LC2W towards MetS from aspects of clinical outcomes and microflora modulation, providing an alternative strategy for people at high risk of MetS.
Trial registration: The study was proactively registered in ClinicalTrial.gov with the registration number of ChiCTR2000031833 on April 09, 2020.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Metabolic syndrome (MetS) is a complex disorder, and its clinical manifestation often comprises abdominal obesity, hyperglycemia, hypertriglyceridemia, lowered high-density lipoprotein cholesterol (HDL-C) levels, and so on [1]. Though the etiology and pathophysiologic mechanisms of MetS are still lack of full understanding and clarity, it is recognized to be a pro-inflammatory and oxidative physiologic state implicated by insulin resistance [2], which confers up soared risk and morbidity of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) [3]. The global prevalence of MetS exhibited a soaring tendency with socioeconomic promotion and living standards improvement. According to the estimation of the International Diabetes Federation (IDF), as much as 25% of the world’s population suffers from MetS [4]. This situation is also serious among Chinese and increases with advanced age. A recent study based on 9258 subjects of the China Health and Retirement Longitudinal Study (CHARLS) datasets indicated the MetS prevalence was about 33.38%, and this figure continuously increased in subjects aged 40–70 years [5]. Liu et al suggested a prevalence of 58.1% in subjects older than 60 years among 2102 subjects [6]. Therefore, intervention with efficacy to alleviate MetS is urgent.
Though ignored in MetS definition, intestinal microbiota might have played a crucial role in the onset and development of MetS according to the last decade’s datasets [7]. Supplement of probiotics is widely accepted as one of the predominant strategies to adjust gut microbiota and to reduce low-grade inflammation states by restoring intestinal microbiota homeostasis and reinforcing the integrity of the intestinal barrier [8]. Several studies have tried to unravel the association of intestinal microbiota with the onset of MetS and explored the potential of probiotics as biotherapeutics for the prevention of MetS. For instance, Lactiplantibacillus plantarum was proven to modulate gut microbiota and metabolites in mice [9], accompanied by an alleviated lipid profile as well as glucose metabolism disorders and reduced inflammation markers associated with MetS [10, 11]. It was found that L. paracasei NL41 could decrease insulin resistance, oxidative stress status, and LPS-induced inflammation by improving the gut microbiota and preserving intestinal integrity, which prevented T2DM [12, 13]. Moreover, Bifidobacterium animalis subsp. lactis Bb12 was reported to ameliorate obesity by enriching beneficial bacteria in the gut [14], and effectively decrease triglyceride concentration and homeostasis model of assessment-insulin resistance (HOMA-IR) while significantly increase high-density lipoprotein-cholesterol and quantitative insulin sensitivity check index (QUICKI) [15].
As one of the well-known probiotic strains, L. casei LC2W was originally isolated in 2001 and its whole genome sequencing was accomplished in 2011 [16]. L. casei LC2W could inhibit Escherichia coli O157:H7 colonization and prevent colitis [17], inhibit the inflammatory response and improve acute lung injury, produce exopolysaccharide (EPS), and exhibit anti-hypertensive bioactivity [18]. Besides, L. casei LC2W exhibited potential for anti-hyperglycemia, for its strong alpha-glucosidase inhibitory activity in vitro [19]. However, no investigation regarding L. casei LC2W on gut microbiome and clinical outcomes has been reported in MetS subjects yet.
In the present study, a randomized, double-blinded, placebo-controlled study was undertaken to investigate the effect of oral administration of L. casei LC2W on glucose and lipid profile components as primary endpoints, as well as on inflammation and oxidation stress biomarkers. Moreover, the effects of L. casei LC2W on gut microbiome composition, cooperation, and function were also investigated. The hypoglycemic and hypolipidemic activity of L. casei LC2W in subjects at high risk of MetS was inferred for its ability to modulate the composition and function of the gut microbiome.
Methods
Study Design
A randomized, double-blinded, placebo-controlled study was carried out in 60 subjects with fasting blood glucose higher than 6.1 mmol/L, but lower than 6.9 mmol/L. Enrolled subjects were randomly assigned to L. casei LC2W or placebo group. Subjects were required to orally take in 6.0 × 1011 colony forming units (CFU) with maltodextrin as the adjuvant or equal weight of maltodextrin for placebo group daily for 6 months. During the study, the dietary intake, sleeping time, physiological exercise, and adverse side reactions of the subjects were recorded weekly with a questionnaire by medical professionals. Subjects forgetting to take in the assigned L. casei LC2W powder or placebo for consecutive 3 days and /or ongoing antibiotic treatment during this study were withdrawn. Twenty-eight subjects in the L. casei LC2W group and 27 subjects in the placebo group accomplished the study (Table 1).
At consumption, subjects took the contents of the sachet directly or with warm water half an hour after the meal. The probiotics powder or placebo were assigned to subjects every 2 months, and the subjects were advised to store the study materials in −20 °C refrigerators throughout the study period.
Subject Recruitment
Eligible subjects included males and females aged 45–65 years with impaired fasting blood glucose (6.1–6.9 mmol/L) and not under any treatment for blood glucose reduction. Exclusion criteria were diagnosis of diabetes; current treatment for diabetes or gastrointestinal symptoms; presence of active diarrhea; current use of pain relievers such as aspirin or paracetamol; use of laxatives or other supplements to improve digestive gastrointestinal function within 2 weeks before the study entry; history of long-term use of probiotics or prebiotics; ongoing use of antihistamines medication, cough medication, or high-dose vitamin C; use of antibiotics within three months prior to study entry; vaccination for upper respiratory tract infection within 6 months or other vaccination within 15 days prior to study entry; alcohol or drug addiction; and pregnant or breast-feeding women. All recruited subjects were instructed to maintain their usual diet but to avoid consumption of other fermented milk, yogurt, prebiotics, and probiotics products during the study. Subjects withdrew from the study if their glucose kept increasing to the extent that medical treatments were needed.
Efficacy Evaluation
All efficacy outcomes except fecal biomarkers were measured at baseline, 3 months, and 6 months after probiotic consumption. The primary outcome was fasting blood glucose. Blood samples were drawn between 8 to 10 a.m. following an overnight fast of at least 12 h to quantify glycemic status, lipid concentrations, biomarkers of inflammation, and oxidative stress. The serum insulin was determined by electrochemiluminescence assay (Roche Diagnostics, Germany), and hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography (Medconn Diagnostics, China). Plasma high-sensitivity C-reactive protein (hs-CRP) concentration was measured by an immunoturbidimetry assay (Goldsite, China). The serum glucose, low-density lipoprotein (LDL), high-density lipoprotein (HDL) cholesterol, total cholesterol (TC), and triglyceride (TG) concentrations were determined by enzymatic kits (Maccura, China); inflammation biomarkers including interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor (TNF-α) were quantified with chemiluminescence methods (Siemens, Germany); oxidative stress biomarkers including superoxide dismutase (SOD) and malondialdehyde (MDA) were determined by spectrophotometric test (Medicom, China).
The oral glucose tolerance test (OGTT) was performed 30 min, 1 h, and 2 h after intake of 75 g of glucose solubilized in 250 mL of warm water. Body weight, height, body mass index (BMI), waist circumference, hip circumference, and waist-to-hip ratio were measured using standard anthropometric methods. Fecal samples were collected at baseline and 3 months post-intervention for short-chain fatty acid measurements with a gas chromatographic method with Agilent 6890N. Subjects were instructed to record their daily food and beverage intake during the 3 days before each visit according to the food models and scales provided. The portion sizes were converted to grams and summarized by food categories. The duration of physical activities in the past week of each visit was also recorded. These data were collected to assess if there was a change in diet and exercise frequency.
Fecal Microbiome Endpoints
Fecal samples collected at baseline, 3 months, and 6 months were used for DNA extraction according to the instruction of E.Z.N.A.® stool DNA Kit (Omega Biotek, Norcross, GA, USA), and 16S rRNA hypervariable V3–V4 region were amplified by a thermocycler PCR system (GeneAmp 9700, ABI, USA) within 20 µL reaction mixture consisting of 4 µL 5 × FastPfu Buffer, 2 μL 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL FastPfu polymerase, and 10 ng template DNA. The PCR products were purified by the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and pooled together with equal mole concentrations, followed by Illumina MiSeq platform (Illumina, San Diego, CA, USA) sequencing which generated 2 × 300 paired-end reads according to standard protocols by Sinotech Genome Technology Co., Ltd. (Shanghai, China).
Statistical Analysis
Demographic and baseline characteristics were summarized to evaluate the significance of the difference between the L. casei LC2W and placebo group. For all efficacy outcomes, continuous variables are reported as means ± SD or median (quartiles), and categorical variables are reported as n (%). Prior to testing, distributional assumptions for the outcomes were assessed and transformations or nonparametric versions of the tests were used if deemed necessary. The group differences at each visit were evaluated using analysis of variance with adjustment for gender for normal distributed continuous outcomes, Kruskal Wallis test for non-normal data, and chi-square test for categorical data. The within-group between every two visits was evaluated using paired t-test for normal distributed continuous outcomes and Wilcoxon signed ranks test for non-normal outcomes. The number and percent of adverse events (AE) and serious adverse events (SAE) were summarized, and the overall AE rate was compared between the two study groups. All efficacy analyses were conducted for subjects who completed the study. The analysis of AE was performed for all subjects. The significance level for statistical tests was set at 0.05. Statistical analyses were performed using the SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Fecal microbiome post-sequencing data were mainly processed with the Mothur software (version v.1.30.1), and the minimum sequencing depth of samples was controlled as 23,984. Usearch (version 7.0) was chosen for operational taxonomic units (OTU) clustering. Bacterial alpha diversity indices Chao 1 were calculated, and Bray-Curtis distance was used to measure community construction according to principal coordinate analysis, comparison between groups at the same visits was performed by Wilcoxon test, while Kruskal Wallis test was within-group between each visit, and the P value was adjusted by Bonferroni method. Linear discriminant analysis effect size (LEfSe) [20] was utilized to illustrate the differences in microbiome composition and functional pathways between groups. PICRUST2 [21] was applied to determine the functional attributions by 16S rRNA OTUs, and Metabolic Pathway Database (MetaCyc) pathways were predicted. Random forest (RF) classification algorithm was employed to efficiently identify key species as well as pathways category that were most important for sample classification between groups by R version 4.2.1. Co-occurrence network analysis was adopted to measure the correlations between species which were found in at least five samples and with total relative abundance larger than 0.1%, and visualization was made by Gephi. Spearman correlation was executed for microbes, pathways, and biomarker associations.
Ethics Statement
The research practices were carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human beings. The study was reviewed and approved by The Institutional Review Board of the Shanghai Nutrition Society. The study was registered with Clinicaltrials.gov (ChiCTR2000031833), and written informed consent was obtained from all subjects prior to their screening and recruitment in the study. Information about all subjects was kept anonymous, and the privacy rights of subjects were always preserved. All institutional safety standards were adhered to.
Data Availability Statement
The high-throughput sequencing data amplified in this study have been deposited in the Sequence Read Archive (SRA) database under the BioProject number: PRJNA896346 (https://www.ncbi.nlm.nih.gov/sra/PRJNA896346).
Results
Of the 60 subjects recruited, mean age was 53.1 years (standard deviation, SD: 4.1 years) and half were men. During the study, three subjects withdrew before the visit at 3 months and another 2 withdrew before the visit at 6 months. The overall dropout rate was 8.3%. All drop-outs were due to personal reasons. Thus, 55 subjects completed the study and were included in the analyses (Table 1). Baseline characteristics were similar between the L. casei LC2W and placebo groups (Table S1). During the study, all subjects complied with dietary restrictions and a 72-h dietary recall and 1-week physical activities of each visit was recorded in Tables S2 and S3.
Anthropometric Measurements
There was no significant difference in anthropometric measurements at baseline (Table S1). However, both body weight (mean change −1.4 kg; 95% CI −2.4, −0.4) and BMI (−0.5 kg/m2; −0.8, −0.1) significantly decreased in response to L. casei LC2W consumption for 6 months (Table S4), and the weight gain of 6 months L. casei LC2W consumption was significantly lower than placebo group (Fig. S1). No significant change was observed in waist circumference, hip circumference, and waist-to-hip ratio (Table S4).
The anthropometric measurements analyses for subgroups of gender exhibited that body weight (female: mean change −1.2 kg; 95% CI −3.3, 1.0; male: mean change −1.5 kg; 95% CI −2.5, −0.5) and BMI (female −0.4 kg/m2; −1.2, 0.3; male: −0.5 kg/m2; −0.8, 0.1) also significantly decreased in response to L. casei LC2W consumption for 6 months. No significant difference was found in anthropometric parameters between female and male subgroups (Table S5).
Blood and Fecal Biomarkers
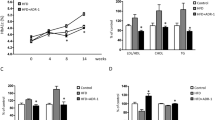
There was no significant difference in all blood biomarkers at baseline. After 3 months of L. casei LC2W consumption, glucose metabolism markers slightly decreased; however, none of the changes was significant. Significant reductions in serum level of fasting glucose (−0.7 mmol/L; 95% confidence interval, CI −0.9, −0.6), glucose at 30 min (−3.1; −3.8, −2.5), 1 h (−2.7; −3.3, −2.1) and 2 h (−1.4; −1.6, −1.1) in OGTT, insulin (−4.7; −11.6, −2.3), and HbA1c (−0.5%; −0.8, −0.2) were observed after 6 months of L. casei LC2W consumption. The levels of all glucose metabolism markers measured were significantly lower than that of the placebo group at 6 months (Fig. 1; Table S6). TG (−0.4 mmol/L; −0.7, −0.2), TC (−0.4 mmol/L; −0.6, −0.3), CRP (−0.3 mg/L; −0.7, −0.1), IL-6 (−0.4 pg/ml; −0.6, −0.2), and MDA (−1.1 nmol/ml; −1.5, −0.6) decreased significantly after 3 months of L. casei LC2W intake. Further decreases in these biomarkers were observed at 6 months. The concentrations of these biomarkers of the L. casei LC2W group were significantly lower than that of the placebo group at 3 months and 6 months after intervention. At 6 months, the L. casei LC2W group also showed significantly lower LDL-cholesterol concentration compared to baseline (−0.5 mmol/L; −0.6, −0.3) and the placebo group. Besides, consumption of L. casei LC2W resulted in significant increases in serum HDL-cholesterol (0.2 mmol/L; 0.1, 0.3) and SOD (18.9 nmol/ml; 13.0, 24.7) at 3 months. Similarly, HDL and SOD concentrations of the L. casei LC2W group were significantly higher than that of the placebo group at 6 months after intervention. There was no significant change in IL-8 and TFN-α at all visits (Table S7).
Effects of L. casei LC2W supplementation on the change of blood biomarkers. Only biomarkers with significant differences are displayed here. Glucose metabolism markers include fasting glucose (a), insulin (b), glycated hemoglobin (c), and glucose tolerance at 2 h (d). Lipid metabolism markers consist of triglyceride (e), total cholesterol (f), HDL-cholesterol (g), and LDL-cholesterol (h). Inflammation and oxidative stress biomarkers involve high-sensitivity CRP (i), interleukin-6 (j), superoxide dismutase (k), and malondialdehyde (l). Blue color system represents for L. casei LC2W group, orange color system stands for placebo group. “*” indicates a significant difference compared to baseline or 3M values, *P < 0.05, **P < 0.01, ***P < 0.001; “#” denotes a significant difference compared to the placebo group, #P < 0.05, ##P < 0.01, ###P < 0.001
As for the subgroups adjust for gender, the majority of the blood biomarker exhibited the consistent results with the adjustment of L. casei LC2W to the whole cohorts, without differences between two genders. However, after 6 months L. casei LC2W consumption, total cholesterol of female subgroup decreased significantly (−0.92 mmol/L; −1.37, −0.46) and was obvious lower than male subgroup (−0.35; −0.51, −0.16), while the level of IL-6 in male subgroup (−0.82 pg/ml; −1.15, −0.49) was significantly lower than that in female subgroup (−0.30; −0.44; −0.16) (Table S7), showing a slightly difference response in subjects intaking of L. casei LC2W.
The fecal concentration of acetic acid (5.0%; 95% CI 1.2, 8.8), butyric acid (4.2%; 2.1, 7.3), and total SCFA (10.3%; 3.4, 16.8) increased significantly after 3 months of L. casei LC2W consumption, and the post-intervention values were significantly higher than that of the placebo group (Fig. 2). There were no significant changes in propionic acid in both study groups.
Effects of L. casei LC2W supplementation on the change of fecal biomarkers. Acetic acid (a), propionic acid (b), butyric acid (c), and total short-chain fatty acid (d). Blue color system represents for L. casei LC2W group; orange color system stands for placebo group. “*” indicates significant different compared to baseline values, *P < 0.05, ** P< 0.01. “#” denotes a significant difference compared to placebo, #P < 0.05
Microbiome Composition and Correlation Profiling
Regarding the bacterial diversity in the fecal samples, there was no significant difference in both alpha and beta diversity between the two groups at different sampling times. For fecal microbiome composition, no significant differences were found between the two groups sampled at both 3 months and 6 months at the phylum level. However, for the overall datasets, LEfSe identified that genus Lacticasebacillus, Veillonella, Fusobacterium, and Haemophilus were enriched after 3-month intervention with L. casei LC2W, while the relative abundance of Parabacterioldes, Alistipes, Pyramidobacter, ClostridiumIV, and unclassified_Ruminococcaceae was significantly higher in the placebo 3 months datasets (LDA score > 2, P < 0.05; Fig. 3b). After 6 months of intervention with L. casei LC2W, the relative abundance of Dorea was significantly improved, while the Desulfovibrionaceae family showed predominance in the placebo 6 months cohort (LDA score > 2, P < 0.05; Fig. 3c). Further OTU level comparisons were executed on 34 major OTUs with relative abundance > 0.5% for microbiome composition analysis. According to the heatmap, there were no significant differences in major OTUs except for Kineothrix OTU43 in both groups at baseline (Fig. 3a). However, it was exhibited that the relative abundance of Bifidobacterium OTU40 and 2 Blautia OTUs (OTU22 and OTU73) was obviously diminished in placebo datasets along sampling time (Kruskal-Wallis test, P < 0.05). In contrast, their relative abundance showed no significant differences after L. casei LC2W intervention, and the relative abundance of Bifidobacterium OTU40 was even elevated at 6 months versus baseline (Fig. 3a). Besides, after 6 months of L. casei LC2W intake, the relative abundance of Lachnospira OTU51 was also significantly increased, while there was no obvious increment in the placebo dataset. For the overall datasets, a Lacticaseibacillus OTU148 was promoted by 3 months of L. casei LC2W intake, while two Bacteroides OTUs (OTU52 and OTU145) as well as Kineothrix OTU43 were increased in 3 months placebo groups (Fig. 3b). For 6 months dataset, Blautia OTU73, Dorea OTU107, and Phascolarctobacterium OTU283 were elevated by intaking L. casei LC2W, while Bacteroides OTU44, Enterobacteriaceae OTU267, and Bilophila OTU209 possessed a high abundance in placebo groups (Fig. 3c). The random forest classification was established, and top 10 OTUs with the highest mean decrease accuracy and mean decrease gini value were exhibited; alike the results obtained by LEfSe, an increase in the relative abundance of Escherichia OTU12 and Agathobacter OTU16, as well as Blautia OTU22, Bifidobacterium OTU40, Bacteroides OTU26, and Anaerostipes OTU265, was selected by the RF model in the feces after 3 months and 6 months of L. casei LC2W intake, respectively. The enrichment of Parabacteroides OTU151, Bacteroides OTU60, and Turicibacter OTU279 was identified in 3 months as well as 6 months placebo datasets (Fig. 3 d and e).
Bacterial heatmap of major 34 OTUs’ relative abundance according to six groups based on L. casei LC2W applied or not and sampling time (a). “*” indicates P < 0.05 versus baseline in the Kruskal-Wallis test adjusted by the Bonferroni method. “#” denotes P < 0.05 versus placebo group by Wilcoxon test. Distinct bacterial features were identified by LEfSe analysis between L. casei LC2W and placebo datasets in 3 months (b) and 6 months (c). The cladogram showed the taxonomical levels from phylum down to genus and the histogram revealed OTU levels. Top 10 OTUs with the highest mean decrease accuracy and Gini scores by Random Forest classification model for 3 months (d) and 6 months (e) datasets. The color of the dots represents the enrichment of OTUs in each group by the median
According to the results of microbiome composition analysis and random forest classification, Blautia and Bifidobacterium OTUs were enriched by L. casei LC2W consumption for both female and male subgroups, while Parabacteroides and Turicibacter OTUs possessed higher relative abundance in female and male subjects in the placebo group, respectively (Fig. S2).
To further explore the microbiome correlation and connection, concurrence networks were established for both the L. casei LC2W and placebo groups at baseline and 6 months (Spearman |r| > 0.5, P < 0.05; Fig. 4). Although the number of nodes in the network constructed by L. casei LC2W datasets is less than those of placebo datasets at both baseline and 6 months, intervention of L. casei LC2W promoted the network complexity as the number of edges increased by 2.16-fold, whereas the edge numbers of placebo group only increased by 1.51-fold. Moreover, the network density was also increased after intake of L. casei LC2W compared to the placebo group, indicating a relatively strong regulating effects on microbiome community after L. casei LC2W intake. Furthermore, the OTUs significantly enriched by L. casei LC2W intake also showed up in the L. casei LC2W co-occurrence pattern and absent in placebo network, with the average weighted degree increased by 2.04-fold, 3.84-fold, 5.25-fold, 6.46-fold, and 14.13-fold for Blautia OTU22, Lachnospira OTU51, Bifidobacterium OTU40, Bacteroides OTU26, and Dorea OTU107, respectively. Notably, Anaerostipes OTU265, which disappeared in L. casei LC2W baseline co-occurrence network, possess a high average weighted degree of 25.24 in the network of fecal microbiota with L. casei LC2W intake for 6 months, suggesting L. casei LC2W intake could not only stimulate the specific beneficial species such as Blautia OTU22, Lachnospira OTU51, Bifidobacterium OTU40, Bacteroides OTU26, and Dorea OTU107 but also enhance their interfere towards microbiome interaction, especially for Anaerostipes OTU265.
Co-occurrence networks established by OTUs appeared in at least five samples with a total relative abundance larger than 0.1% for the baseline placebo group (a), baseline L. casei LC2W group (b), 6 months placebo group (c), and 6 months L. casei LC2W group (d). The nodes represent 16S rRNA OTUs with the size reflecting the average weighted degree in the co-occurrence pattern. The edges represent the correlation between nodes, and the thickness stands for Spearman correlation coefficients; the edges’ color indicates the correlation was positive (orange) or negative (blue)
Microbiome Function Prediction
According to the MetaCyc pathway abundance table generated by PICRUST2 for OTUs in each dataset, a total of 4 pathways were significantly enriched in the L. casei LC2W group after both 3 months and 6 months of intaking (LDA score > 2 and p < 0.05), including L-methionine and S-adenosyl-L-methionine biosynthesis (MET-SAM-PWY, PWY-5347, and HOMOSER-METSYN-PWY) and fatty acid degradation (FAO-PWY) (Fig. 5b). Besides, L-tryptophan biosynthesis (PWY-6629) was significantly elevated by L. casei LC2W intake for 3 months, while lactose and galactose degradation (LACTOSESCAT-PWY), as well as teichoic acid biosynthesis (TEICHOICACID-PWY), exhibited promotion after 6 months consumption (Fig. 5b). Of note, glucose and glucose-1-phosphate degradation (GLUCOSE1PMETAB-PWY), which occurred as the major characteristic pathway in the placebo group at baseline (Fig. 5a), was increased by L. casei LC2W intake, and its relative abundance in L. casei LC2W group has exceeded placebo groups at 3 months and 6 months. Although three sugar acid degradation pathways including GLUCUROCAT-PWY, GLUCUROCAT-PWY, and GALACTUROCAT-PWY were overabundant in the baseline placebo group (LDA score > 2 and P < 0.05), their predominance in placebo group were disappeared, as 3 months of L. casei LC2W intake enhanced the first two pathways, while 6 months of L. casei LC2W intake promoted the last pathway significantly, even surpass the placebo group (Kruskal-Wallis test, P < 0.05; Fig. 5c). As for homolactic fermentation (ANAEROFRUCAT-PWY) and biotin biosynthesis (BIOTIN-BIOSYNTHESIS-PWY) which were prevailing in the placebo group at baseline, 6 months intake of L. casei LC2W has significantly enriched these two pathways and surpassed the placebo group (Kruskal-Wallis, P < 0.05; Fig. 5c). When it comes to the placebo group, three pathways involving sulfur compound metabolism (SULFATE_CYS_PWY and SO4ASSIM_PWY) and glycosaminoglycan degradation (PWY-6572) possess greater proportions in both 3 and 6 months.
Top 10 pathways with the highest variable importance selected by random forest classification model in baseline (a). The color of the dots represents the predicted pathways that were predominant in L. casei LC2W (blue) and placebo (orange), respectively. LEfSe histogram depicting discriminatory pathways between the L. casei LC2W (blue) and placebo (orange) cohorts in 3 months and 6 months (b). The median proportions in pathways differences between the two datasets at different times (c). “*” indicates P < 0.05 versus baseline in the Kruskal-Wallis test adjusted by the Bonferroni method. “#” denotes P < 0.05 versus placebo group by Wilcoxon test
Association of Bacterial OTUs with Biomarkers and Function
Spearman correlation analysis was carried out to figure out the exact correlation between the bacterial OTUs, pathways, and biomarkers that differed in L. casei LC2W and placebo groups (Fig. 6). The relative abundance of Anaerostipes OTU265, Bifidobacterium OTU40, and Lacticaseibacillus OTU148 which were enriched by L. casei LC2W intake was positively correlated with L-methionine and S-adenosyl-L-methionine biosynthesis pathways, while the Parabacteroide OTU151, Bacteroides OTU52, and Bilophila OTU209 enriched in the placebo group exhibited opposite trend (Fig. 6a). As for fatty acid degradation, the majority of OTUs with higher relative abundance in the placebo dataset possessed a negative correlation trend, while Anaerostipes OTU265 and Lacticaseibacillus OTU148, which were enriched in L. casei LC2W group, showed promoting effects. A total of 6 OTUs predominant in the L. casei LC2W group actively correlated with lactose and galactose degradation; however, there were only one OTU enriched in the placebo group showing a positive correlation and five of the other placebo-enriched OTUs exhibiting a negative tendency with this pathway. Three OTUs with higher relative abundance after L. casei LC2W intake including Blautia OTU22, Anaerostipes OTU265, and Bifidobacterium OTU40 were actively related to fermentation and glucose degradation pathways. However, no positive relationship was found between fermentation pathway and OTUs with higher relative abundance in the placebo group. A total of four OTUs enriched in the L. casei LC2W group positively correlated with an increased abundance of teichoic acid and pyrimidine deoxyribonucleotide biosynthesis (P < 0.001). On the contrary, OTUs promoted in placebo datasets do not show a close connection between these two pathways except for Turicibacter OTU279. As for the pathways with high levels in the placebo group, microbes enriched in L. casei LC2W group exhibited a weaker connection or opposite negative trend compared to OTUs promoted in the placebo group.
Association between major discriminatory microbe OTUs and specific pathways of differences (a). The relationship of 15 OTUs and 19 MetaCyc pathways in the L. casei LC2W and placebo cohorts was reflected by heatmap with orange and cyan indicating positive and negative Spearman correlation coefficients, respectively. Orange and blue shades represent the microbes, and the pathways were enriched in the placebo and L. casei LC2W groups, respectively. “**” and “*” denote P < 0.01 and P < 0.05, respectively. Association between major discriminatory microbe OTUs and 16 biomarkers (b). The relationship of 15 OTUs and 16 biomarkers in the L. casei LC2W and placebo cohorts were reflected by heatmap with orange and cyan indicating positive and negative Spearman correlation coefficients, respectively. Orange and blue shades represent the microbes that were enriched in the placebo and L. casei LC2W groups, respectively. “**” and “*” denote P < 0.01 and P < 0.05, respectively
Bacterial OTUs promoted by L. casei LC2W intake including Bacteroide OTU26, Lachnospira OTU51, Lacticaseibacillu OTU148, Dorea OTU107, and Anaerostipes OTU265 exhibited negative correlations with one or more glucose metabolism biomarkers. However, bacterial OTUs with higher abundance in placebo groups such as Bacteroides OTUs (OTU145 and OTU44), and Turicibacter OTU279 exhibited positive trends with these biomarkers (Fig. 6b). As for lipids metabolism markers, L. casei LC2W-enriched OTUs were negatively correlated with triglyceride and positively correlated with HDL-cholesterol, with Lacticaseibacillus OTU148 displaying negative effects with triglyceride, total, and LDL-cholesterol, while most of the placebo-enriched OTUs exhibited the opposite trends although not all of the correlation showed significances. A similar situation was also found in biomarkers involving inflammation, as most of the bacterial OTUs promoted by L. casei LC2W intake were negatively correlated with pro-inflammatory factors while bacterial OTUs abundant in the placebo group showed positive correlation. When it comes to malondialdehyde, a biomarker related to oxidative stress, three OTUs including Bacteroide OTU26, Lachnospira OTU51, and Dorea OTU107 exhibited a pronounced negative correlation in the L. casei LC2W group, while only Parabacteroide OTU151 revealed a negative relationship in the placebo group.
Adverse Events
There were 28 adverse events during the study (Table S8). However, no significant difference was found between the two study groups in the overall AE rate (P = 0.438). None of the adverse events was judged to correlate to the tested the probiotic product by medical professionals. No serious AE was recorded during the study.
Discussion
Metabolic syndrome (MetS), which manifests as central obesity together with high levels in triacylglycerols, blood pressure, fasting plasma glucose, and low level in high-density lipoprotein cholesterol, leads to an increasing risk of both type 2 diabetes mellitus and cardiovascular disease. In the current study, subjects aged 45–65 years with high risks of MetS were administered lyophilized L. casei LC2W powder with maltodextrin as an adjuvant for 6 months, to explore its amelioration effects on MetS from both clinical and microbiome indicators.
As for the clinical outcomes, intake of L. casei LC2W could decrease the body weights as well as BMI index (Table S4), and reduce all six glucose metabolism markers measured and lipid markers while elevating HDL-cholesterol of subjects of high risk of MetS (Fig. 1; Table S6). Besides, L. casei LC2W significantly reduced the inflammation markers including hsCRP and IL-6. IL-6 was reported to induce insulin resistance by inhibiting transcription or reducing phosphorylation of insulin receptor substrate-1 [22], while elevated hsCRP was a prestigious biomarker in inflammation and associated tightly with cardiovascular risk [23]. Hence, L. casei LC2W could benefits subjects with high risks of MetS via reducing inflammation intensity. Meanwhile, intake of L. casei LC2W also decreased the serum MDA level while increasing serum SOD level, significantly. MDA is the end product of lipid peroxidation and hallmark of elevated oxidative stress, while SOD could help eliminate harmful peroxides generated during metabolic processes [24, 25]. Their changes indicated that intake of L. casei LC2W could effectively reduce oxidative stress and hence improve glucose and lipid metabolism in the body. Moreover, intake of L. casei LC2W also significantly increased the fecal content of SCFAs including acetic acid and butyric acid (Fig. 2), which exhibited significant negative correlation with some of the metabolism and inflammation markers including 2-h-glucose tolerance, LDL-cholesterol, and hsCRP (Table S9), and positively correlated with Blautia OTU73 and Lactobacillus OTU28 (Table S10) and fermentation pathway (Table S11). SCFAs could serve as signaling molecules between the gut microbiome and the host [26] and play a key role in the homeostasis of glucose metabolism by reducing the oxidative stress of β-cells in the pancreatic islet, increasing insulin release, and reducing the expression of pro-inflammatory cytokines and anti-lipolysis [27].
Besides clinical biomarkers, we also analyze the microbiome composition, interaction, and function to infer the ability of glucose and lipid modulation of L. casei LC2W from a microbiome perspective. Results from previous reports suggested that different OTUs within the same genus might play discriminate roles with some providing protective effects while others exerting detrimental impacts to the host health [28]. Thus, in our study, the priority on analysis of microbiota composition was focused on OTU level, and we found that L. casei LC2W application not only increased the relative abundance of specific OTUs but also enhanced their interference towards microbiome interaction (Fig. 4). It is shown that the Lacticaseibacillus OTU148 was enriched after L. casei LC2W intervention, and L. casei LC2W intake could also enhance the relative abundance of Bifidobacterium OTU40, members of which were reported to effectively lower cholesterol and display anti-inflammatory activity in high-fat diet feed mouse model [29]. Besides, L. casei LC2W intake promoted the relative abundance of Anaerostipe OTU265, which was reported to protect animals from food anaphylaxis in inbred germ-free mice and thus be considered a candidate of the next generation probiotics [30]. According to the correlation analysis, the above three OTUs exhibited negative trends towards IL-6 (with Lacticaseibacillus OTU148 exhibited significances; Fig. 6b) and were positively correlated with L-methionine and S-adenosyl-L-methionine biosynthesis pathways (Fig. 6a). L-methionine was reported to improve intestinal barrier integrity [31], while S-adenosyl-L-methionine could play diversified roles in health maintenance, including enhanced energy production in cells as well as insulin sensitivity [32,33,34]. Thus, the underlying mechanism for L. casei LC2W to ameliorate the risk of MetS might be correlate with its anti-inflammatory activity. In addition, Anaerostipes OTU265 and Lacticaseibacillus OTU148 possess a positive correlation trend with fatty acid degradation (Fig. 6a), and Lacticaseibacillus OTU148 display negative effects with triglyceride (P < 0.05; Fig. 6b), total, and LDL-cholesterol, suggesting a possible pathway of L. casei LC2W in decreasing serum free fatty acid and triglycerides.
Previous studies claimed that depletion of Blautia species (especially Blautia luti and Blautia wexlerae) was positively correlated with insulin resistance in obese individuals [35], and oral administration of Blautia to mice could eliminate the symptoms of both obesity and diabetes [36]. Due to their antibacterial activity, anti-inflammatory effects, and inverse correlation with aging, members of Blautia were proposed as the next generation of probiotics [37, 38]. Members in the Dorea genus, most of which exhibited anti-inflammatory activity in vivo, were often found tightly associated with Blautia as the gas produced by Dorea from carbohydrates could be further utilized by Blautia [39]. A similar situation was also observed in subjects with L. casei LC2W intake in this study, as the abundance of two Blautia OTUs and Dorea were promoted, simultaneously (Fig. 2a and c). According to the correlation analysis, three OTUs with higher relative abundance after L. casei LC2W intake including Blautia OTU22, Anaerostipes OTU265, and Bifidobacterium OTU40 were actively related to acids fermentation and glucose degradation, and a total of 6 OTUs predominant in L. casei LC2W group actively correlated with lactose and galactose degradation (Fig. 6a). Moreover, Lacticaseibacillus OTU148, Dorea OTU107, and Anaerostipes OTU265 exhibited negative correlations with one or more glucose metabolic biomarkers (Fig. 6b). Together, it is indicated that L. casei LC2W intake boosted pathways of homolactic fermentation, glucose, and lactose degradation, which helps to decrease the serum glucose. Lacticaseibacillus casei LC2W consumption also enhanced mixed acid fermentation and its principal products short-chain fatty acids, which was proved to be negatively correlated with obesity degree [40].
Interestingly, L. casei LC2W seemed to exert different effects within Bacteroidetes OTUs, with the relative abundance of Bacteroides OTU26 promoted, while three other Bacteroidetes OTUs (OTU44, OTU52, and OTU145) decreased (Fig. 2). Moreover, Bacteroides OTU26 was negatively correlated to glucose metabolism and MDA, while the three decreased Bacteroidetes OTUs exhibited a contrasting trend, and some of them were positively correlated with glucose as well as inflammatory biomarkers (Fig. 6b). Therefore, one of the underlying pathways for the hypoglycemic effect of L. casei LC2W might stem from the modulation of the Bacteroides in the gut microbiome composition. Controversial roles regarding Bacteroides species to their host health have been reported in the last decade. Li et al found that Bacteroides were associated with T2DM risk in obese individuals by promoting IL-17-producing cell expansion in the peripheral blood [41], and were prevalent in patients with colitis [42, 43]. However, Bacteroides stercoris was infrequently isolated in clinical samples and with less apparent association with diseases [44], and showed a positive correlation with lower diastolic blood pressure [45]. As Bacteroides OTU26 was most closely related to Bacteroides stercoris, its effects in MetS alleviation need to be further verified.
In contrast to the placebo group, intake of L. casei LC2W reduced the relative abundance of Bilophila OTU209 belonging to the family Desulfovibrionaceae; Bilophila OTU209 exhibited a negative correlation with L-methionine and S-adenosyl-L-methionine biosynthesis pathways, and showed significant positive correlation with TNFα (Fig. 6). A higher relative abundance of family Desulfovibrionaceae has been reported in obese humans [46] and mice [47], which was also identified as an important endotoxin producer in constipation patients and could aggravate the symptoms in these patients by reducing intestinal hormone secretion and destroying intestinal integrity [48]. Furthermore, administration of L. casei LC2W also reduced the relative abundance of Turicibacter OTU279, which exhibited a tight correlation with glucose and HbA1c concentrations (Fig. 6b). Turicibacter has been reported to express an analog protein to serotonin transporter with strong pro-inflammatory activity [49, 50], which might contribute to the development of MetS.
Overall, this study is the first clinical study to investigate the effects of L. casei LC2W on both the clinical indicators including glucose and lipids metabolism, and the gut microbiome profile among subjects at high risk of MetS. Although previous studies have reported that probiotics or synbiotics administration could alleviate the MetS, the subjects’ number, and phenotypic indicators were limited compared to this study [51]. Besides, although some studies involve the variation of both clinical and microbiome indicators at the same time, the OTU level analysis, functional analysis, and correlation analysis between indicators were ignored [52, 53]. Our study claimed that intake of L. casei LC2W could significantly decrease body weight and BMI, reduce glucose and lipid levels in serum, and eliminate the pro-inflammatory cytokines and oxidative stress compared to the placebo group. The amelioration of MetS by L. casei LC2W might stem from its ability to enhance the relative abundance of beneficial bacteria and their interference towards microbiome interaction, boosting the predicted glucose and lipid degradation pathways in the gut microbiota (Fig. 7). Our study has several strengths; for instance, most clinical studies pay attention to the microbiota shifts in genus level, ignoring the functional discrepancy of OTUs within the same genus, while we focus more on OTU level analysis except for genus level. Besides, in addition to the change in the relative abundance of different bacterial taxa, the interaction among the OTUs was also verified.
Limitations and Innovations
In the present study, the effects of probiotics on subjects with high MetS risk over 6 months employing serum biomarkers, intestinal microbiome, and their predicted function as the major endpoints were intensively investigated. The correlation between different endpoints has also been dig out, which is helpful for the follow-up mechanism research. However, the analysis of SCFAs was only conducted at baseline and 3 months. Besides, the function of bacterial taxa enriched or diminished by L. casei LC2W administration needs to be verified.
Conclusion
As a conclusion, in the randomized, double-blinded, placebo-controlled study, intake of L. casei LC2W for 6 months could significantly improve the symptoms in subjects with a high risk of MetS, including fasting blood glucose, serum lipid level, and BMI. Intake of L. casei LC2W could also ameliorate the inflammatory intensity and oxidative stress, and elevate SCFAs production in the feces. The gut microbiota shifted to a composition with a relatively high abundance of bacterial OTUs belonging to Lacticaseibacillus, Bifidobacterium, Dorea, Blautia, and their interaction with other gut microbes was boosted as well, which was beneficial in improving chronic inflammation, decreasing serum glucose and lipids, thus alleviating MetS, while the relative abundance of bacterial OTUs belongs to Bilophila, Turicibacter, etc., was decreased.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
Abbreviations
- L. casei :
-
lacticaseibacillus casei
- MetS:
-
Metabolic syndrome
- SCFAs:
-
Short-chain fatty acids
- HDL-C:
-
High-density lipoprotein cholesterol
- T2DM:
-
Type 2 diabetes mellitus
- CVD:
-
Cardiovascular disease
- CHARLS:
-
China Health and Retirement Longitudinal Study
- LPS:
-
Lipopolysaccharide
- HOMA-IR:
-
Homeostasis model of assessment-insulin resistance
- QUICKI:
-
Quantitative insulin sensitivity check index
- EPS:
-
Exopolysaccharide
- CFU:
-
Colony forming units
- FBG:
-
Fasting blood glucose
- HbA1c:
-
Hemoglobin A1c
- hs-CRP:
-
High-sensitivity C-reactive protein
- LDL-C:
-
Low-density lipoprotein cholesterol
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- IL-6:
-
Interleukin-6
- IL-8:
-
Interleukin-8
- TNF-α:
-
Tumor necrosis factor
- SOD:
-
Superoxide dismutase
- MDA:
-
Malondialdehyde
- OGTT:
-
Oral glucose tolerance test; BMI: body mass index
- AE:
-
Adverse events
- SAE:
-
Serious adverse events
- OTU:
-
Operational taxonomic units
- LEfSe:
-
Linear discriminant analysis effect size
- RF:
-
Random forest
- PICRUSt2:
-
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2
References
Samson SL, Garber AJ (2014) Metabolic syndrome. Endocrinol Metab Clin North Am 43(1):1–23. https://doi.org/10.1016/j.ecl.2013.09.009
McCracken E, Monaghan M, Sreenivasan S (2018) Pathophysiology of the metabolic syndrome. Clin Dermatol 36(1):14–20. https://doi.org/10.1016/j.clindermatol.2017.09.004
Sookoian S, Pirola CJ (2011) Metabolic syndrome: from the genetics to the pathophysiology. Curr Hypertens Rep 13(2):149–157. https://doi.org/10.1007/s11906-010-0164-9
O’Neill S, O’Driscoll L (2015) Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 16(1):1–12. https://doi.org/10.1111/obr.12229
Ge H, Yang Z, Li X et al (2020) The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci Rep 10(1):20034. https://doi.org/10.1038/s41598-020-77184-x
Liu M, Wang J, Jiang B et al (2013) Increasing prevalence of metabolic syndrome in a Chinese elderly population: 2001–2010. PLoS One 8(6):e66233. https://doi.org/10.1371/journal.pone.0066233
Dabke K, Hendrick G, Devkota S (2019) The gut microbiome and metabolic syndrome. J Clin Invest 129(10):4050–4057. https://doi.org/10.1172/JCI129194
Wieërs G, Belkhir L, Enaud R et al (2020) How probiotics affect the microbiota. Front Cell Infect Microbiol 9:454. https://doi.org/10.3389/fcimb.2019.00454
Axling U, Olsson C, Xu J et al (2012) Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab (Lond) 9(1):105. https://doi.org/10.1186/1743-7075-9-105
Zhao L, Shen Y, Wang Y et al (2022) Lactobacillus plantarum S9 alleviates lipid profile, insulin resistance, and inflammation in high-fat diet-induced metabolic syndrome rats. Sci Rep 12(1):15490. https://doi.org/10.1038/s41598-022-19839-5
Li X, Wang N, Yin B et al (2016) Lactobacillus plantarum X1 with α-glucosidase inhibitory activity ameliorates type 2 diabetes in mice. RSC Adv 6:63536–63547. https://doi.org/10.1039/C6RA10858J
Zeng Z, Yuan Q, Yu R et al (2019) Ameliorative effects of probiotic Lactobacillus paracasei NL41 on insulin sensitivity, oxidative stress, and beta-cell function in a type 2 diabetes mellitus rat model. Mol Nutr Food Res 63(22):e1900457. https://doi.org/10.1002/mnfr.201900457
Zeng Z, Guo X, Zhang J et al (2021) Lactobacillus paracasei modulates the gut microbiota and improves inflammation in type 2 diabetic rats. Food Funct 12(15):6809–6820. https://doi.org/10.1039/d1fo00515d
Mao K, Gao J, Wang X et al (2022) Bifidobacterium animalis subsp. lactis BB-12 has effect against obesity by regulating gut microbiota in two phases in human microbiota-associated rats. Front Nutr 8:811619. https://doi.org/10.3389/fnut.2021.811619
Mohammadi-Sartang M, Bellissimo N, Totosy de Zepetnek JO et al (2018) The effect of daily fortified yogurt consumption on weight loss in adults with metabolic syndrome: a 10-week randomized controlled trial. Nutr Metab Cardiovasc Dis 28(6):565–574. https://doi.org/10.1016/j.numecd.2018.03.001
Chen C, Ai L, Zhou F et al (2011) Complete genome sequence of the probiotic bacterium Lactobacillus casei LC2W. J Bacteriol 193(13):3419–3420. https://doi.org/10.1128/JB.05017-11
Wang G, Zhang Y, Song X et al (2019) Lactobacillus casei LC2W can inhibit the colonization of Escherichia coli O157:H7 in vivo and reduce the severity of colitis. Food Funct 10(9):5843–5852. https://doi.org/10.1039/c9fo01390c
Song X, Xiong Z, Kong L et al (2018) Relationship between putative eps genes and production of exopolysaccharide in Lactobacillus casei LC2W. Front Microbiol 9:1882. https://doi.org/10.3389/fmicb.2018.01882
Fan W, Wu Z, Ji H, Guo B (2012) Comparison of alpha-glucosidase inhibitory effect of different Lactobacillus casei strains. Food Ferment Ind 38(4):29–33. http://sf1970.cnif.cn/EN/Y2012/V38/I04/29
Segata N, Izard J, Waldron L et al (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12(6):R60. https://doi.org/10.1186/gb-2011-12-6-r60
Douglas GM, Maffei VJ, Zaneveld JR et al (2020) PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 38(6):685–688. https://doi.org/10.1038/s41587-020-0548-6
Weigert C, Hennige AM, Lehmann R et al (2006) Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem 281(11):7060–7067. https://doi.org/10.1074/jbc.M509782200
Aday AW, Ridker PM (2019) Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front Cardiovasc Med 6:16. https://doi.org/10.3389/fcvm.2019.00016
Weismann D, Hartvigsen K, Lauer N et al (2011) Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478(7367):76–81. https://doi.org/10.1038/nature10449
Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim) 12(3):88–93
Tanase DM, Gosav EM, Neculae E et al (2020) Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients 12(12):3719. https://doi.org/10.3390/nu12123719
Mandaliya DK, Seshadri S (2019) Short chain fatty acids, pancreatic dysfunction and type 2 diabetes. Pancreatology 19(2):280–284. https://doi.org/10.1016/j.pan.2019.01.021
Wilkins LJ, Monga M, Miller AW (2019) Defining dysbiosis for a cluster of chronic diseases. Sci Rep 9(1):12918. https://doi.org/10.1038/s41598-019-49452-y
Kim YT, Kim CH, Kwon JG et al (2022) In vivo trial of Bifidobacterium longum revealed the complex network correlations between gut microbiota and health promotional effects. Front Microbiol 13:886934. https://doi.org/10.3389/fmicb.2022.886934
Feehley T, Plunkett CH, Bao R et al (2019) Healthy infants harbor intestinal bacteria that protect against food allergy. Nat Med 25(3):448–453. https://doi.org/10.1038/s41591-018-0324-z
Chen Y, Li D, Dai Z et al (2014) L-methionine supplementation maintains the integrity and barrier function of the small-intestinal mucosa in post-weaning piglets. Amino Acids 46(4):1131–1142. https://doi.org/10.1007/s00726-014-1675-5
Jin CJ, Park HK, Cho YM et al (2007) S-adenosyl-L-methionine increases skeletal muscle mitochondrial DNA density and whole body insulin sensitivity in OLETF rats. J Nutr 137(2):339–344. https://doi.org/10.1093/jn/137.2.339
Moon MK, Kim M, Chung SS et al (2010) S-Adenosyl-L-methionine ameliorates TNF alpha-induced insulin resistance in 3T3-L1 adipocytes. Exp Mol Med 42(5):345–352. https://doi.org/10.3858/emm.2010.42.5.036
Kanai M, Mizunuma M, Fujii T, Iefuji H (2017) A genetic method to enhance the accumulation of S-adenosylmethionine in yeast. Appl Microbiol Biotechnol 101(4):1351–1357. https://doi.org/10.1007/s00253-017-8098-7
Benítez-Páez A, Gómez Del Pugar EM, López-Almela I et al (2020) Depletion of Blautia species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems 5(2):e00857-19. https://doi.org/10.1128/mSystems.00857-19
Hosomi K, Saito M, Park J et al (2022) Oral administration of Blautia wexlerae ameliorates obesity and type 2 diabetes via metabolic remodeling of the gut microbiota. Nat Commun 13(1):4477. https://doi.org/10.1038/s41467-022-32015-7
Horigome A, Hashikura N, Yoshida K et al (2022) 2′-Fucosyllactose increases the abundance of Blautia in the presence of extracellular fucosidase-possessing bacteria. Front Microbiol 13:913624. https://doi.org/10.3389/fmicb.2022.913624
Liu X, Mao B, Gu J et al (2021) Blautia—a new functional genus with potential probiotic properties? Gut Microbes 13(1):1–21. https://doi.org/10.1080/19490976.2021.1875796
Rajilić-Stojanović M, Jonkers DM, Salonen A et al (2015) Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena? Am J Gastroenterol 110(2):278–287. https://doi.org/10.1038/ajg.2014.427
Ilyés T, Silaghi CN, Crăciun AM (2022) Diet-related changes of short-chain fatty acids in blood and feces in obesity and metabolic syndrome. Biology (Basel) 11(11):1556. https://doi.org/10.3390/biology11111556
Li Y, Yang Y, Wang J et al (2022) Bacteroides ovatus-mediated CD27- MAIT cell activation is associated with obesity-related T2D progression. Cell Mol Immunol 19(7):791–804. https://doi.org/10.1038/s41423-022-00871-4
Gustafsson RJ, Ohlsson B, Benoni C et al (2012) Mucosa-associated bacteria in two middle-aged women diagnosed with collagenous colitis. World J Gastroenterol 18(14):1628–1634. https://doi.org/10.3748/wjg.v18.i14.1628
Nomura K, Ishikawa D, Okahara K et al (2021) Bacteroidetes species are correlated with disease activity in ulcerative colitis. J Clin Med 10(8):1749. https://doi.org/10.3390/jcm10081749
Otte E, Nielsen HL, Hasman H, Fuglsang-Damgaard D (2017) First report of metronidazole resistant, nimD-positive, Bacteroides stercoris isolated from an abdominal abscess in a 70-year-old woman. Anaerobe 43:91–93. https://doi.org/10.1016/j.anaerobe.2016.12.010
Gaundal L, Myhrstad MCW, Rud I et al (2022) Gut microbiota is associated with dietary intake and metabolic markers in healthy individuals. Food Nutr Res 66. https://doi.org/10.29219/fnr.v66.8580. https://doi.org/10.29219/fnr.v66.8580
Xiao S, Fei N, Pang X et al (2014) A gut microbiota-targeted dietary intervention for amelioration of chronic inflammation underlying metabolic syndrome. FEMS Microbiol Ecol 87(2):357–367. https://doi.org/10.1111/1574-6941.12228
Zhang C, Zhang M, Wang S et al (2010) Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4(2):232–241. https://doi.org/10.1038/ismej.2009.112
Zhuang M, Shang W, Ma Q et al (2019) Abundance of probiotics and butyrate-production microbiome manages constipation via short-chain fatty acids production and hormones secretion. Mol Nutr Food Res 63(23):e1801187. https://doi.org/10.1002/mnfr.201801187
Fung TC, Vuong HE, Luna CDG et al (2019) Intestinal serotonin and fluoxetine exposure modulate bacterial colonization in the gut. Nat Microbiol 4(12):2064–2073. https://doi.org/10.1038/s41564-019-0540-4
Joly A, Leulier F, De Vadder F (2021) Microbial modulation of the development and physiology of the enteric nervous system. Trends Microbiol 29(8):686–699. https://doi.org/10.1016/j.tim.2020.11.007
Barreto FM, Colado Simão AN, Morimoto HK et al (2014) Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition 30(7–8):939–942. https://doi.org/10.1016/j.nut.2013.12.004
Kanazawa A, Aida M, Yoshida Y et al (2021) Effects of synbiotic supplementation on chronic inflammation and the gut microbiota in obese patients with type 2 diabetes mellitus: a randomized controlled study. Nutrients 13:558. https://doi.org/10.3390/nu13020558
Tenorio-Jiménez C, Martínez-Ramírez MJ, Del Castillo-Codes I et al (2019) Lactobacillus reuteri V3401 reduces inflammatory biomarkers and modifies the gastrointestinal microbiome in adults with metabolic syndrome: the PROSIR study. Nutrients 11(8):1761. https://doi.org/10.3390/nu11081761
Acknowledgements
The authors would like to thank Dr. Li Zhang and his colleagues from Sprim China for their excellent work in coordinating the study, and Raison Testing Lab for performing the biomarkers test.
Funding
The Natural Science Foundation of Shanghai, 23ZR1414400, 23ZR1414400, 23ZR1414400, 23ZR1414400, National Key Research and Development Program of China, 2022YFD2100704, 2022YFD2100704, The Shanghai State-owned Assets Supervision and Administration Commission Enterprise Innovation Development and Capacity Enhancement Program, No. 2022013.
Author information
Authors and Affiliations
Contributions
WDQ, WXH, HJ, and WZJ contributed to the design of the clinical study. WDQ, WXH, and WZJ contributed to the interpretation of the data. WDQ wrote the main manuscript and prepared figures, WZJ and all other authors gave critical comments and revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The research practices were carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human beings, and the study was reviewed and approved by The Institutional Review Board of the Shanghai Nutrition Society. The study was registered with Clinicaltrials.gov (ChiCTR2000031833), and written informed consent was obtained from all subjects prior to their screening and recruitment in the study.
Consent for Publication
All authors have reviewed and approved the content of this manuscript for publication.
Competing Interests
All the authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Wang, X., Han, J. et al. Effect of Lacticaseibacillus casei LC2W Supplementation on Glucose Metabolism and Gut Microbiota in Subjects at High Risk of Metabolic Syndrome: A Randomized, Double-blinded, Placebo-controlled Clinical Trial. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10312-5
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10312-5