Abstract
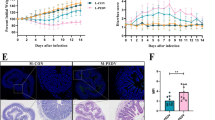
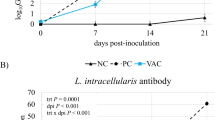
Porcine epidemic diarrhea virus (PEDV) infection results in significant mortality among newborn piglets, leading to substantial economic setbacks in the pig industry. Short-chain fatty acids (SCFA), the metabolites of intestinal probiotics, play pivotal roles in modulating intestinal function, enhancing the intestinal barrier, and bolstering immune responses through diverse mechanisms. The protective potential of Lactobacillus delbrueckii, Lactobacillus johnsonii, and Lactococcus lactis was first noted when administered to PEDV-infected piglets. Histological evaluations, combined with immunofluorescence studies, indicated that piglets receiving L. lactis displayed less intestinal damage, with diminished epithelial cell necrosis and milder injury levels. Differences in immunofluorescence intensity revealed a significant disparity in antigen content between the L. lactis and PEDV groups, suggesting that L. lactis might suppress PEDV replication, the intestine. We then assessed short-chain fatty acid content through targeted metabolomics, finding that acetate levels markedly varied from other groups. This protective impact was confirmed by administering acetate to PEDV-infected piglets. Data suggested that piglets receiving acetate exhibited resistance to PEDV. Flow cytometry analyses were conducted to evaluate the expression of innate and adaptive immune cells in piglets. Sodium acetate appeared to bolster innate immune defenses against PEDV, marked by elevated NK cell and macrophage counts in mesenteric lymph nodes, along with increased NK cells in the spleen and macrophages in the bloodstream. Acetic acid was also found to enhance the populations of CD8+ IFN-γ T cells in the blood, spleen, and mesenteric lymph, CD4+ IFN-γ T cells in mesenteric lymph nodes and spleen, and CD4+ IL-4+T cells in the bloodstream. Transcriptome analyses were carried out on the jejunal mucosa from piglets with PEDV-induced intestinal damage and from healthy counterparts with intact barriers. Through bioinformatics analysis, we pinpointed 189 significantly upregulated genes and 333 downregulated ones, with the PI3K-AKT, ECM-receptor interaction, and pancreatic secretion pathways being notably enriched. This transcriptomic evidence was further corroborated by western blot and qPCR. Short-chain fatty acids (SCFA) were found to modulate G protein-coupled receptor 41 (GPR41) and 43 (GPR43) in porcine intestinal epithelial cells (IPEC-J2). Post-acetic acid exposure, there was a notable upsurge in the ZO-1 barrier protein expression in IPEC-J2 compared to the unexposed control group (WT), while GPR43 knockdown inversely affected ZO-1 expression. Acetic acid amplified the concentrations of phosphorylated PI3K and AKT pivotal components of the PI3K/AKT pathway. Concurrently, the co-administration of AKT agonist SC79 and PI3K inhibitor LY294002 revealed acetic acid’s role in augmenting ZO-1 expression via the P13K/AKT signaling pathway. This study demonstrates that acetic acid produced by Lactobacillus strains regulates intestinal barrier and immune functions to alleviate PEDV infection. These findings provide valuable insights for mitigating the impact of PEDV in the pig industry.










Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this work are available within the article. Raw data that supports the findings are available upon reasonable request.
References
Wood EN (1977) An apparently new syndrome of porcine epidemic diarrhoea 100(12):243–4. https://doi.org/10.1136/vr.100.12.243
Jung K, Saif LJ, Wang Q (2020) Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control. Virus Res 286:198045. https://doi.org/10.1016/j.virusres.2020.198045
Turlewicz-Podbielska H, Pomorska-Mól MJVS (2021) Porcine coronaviruses: overview of the state of the art. Virol Sin 36(5):833–851. https://doi.org/10.1007/s12250-021-00364-0
Sekhon S, Nguyen P, Ahn J, Lee K, Lee L, Kim S et al (2016) Porcine epidemic diarrhea (PED) infection, diagnosis and vaccination: a mini review. Toxicol Environmen Health Sci 8(5):277–89. https://doi.org/10.1007/s13530-016-0287-8
Pensaert M, De Bouck P (1978) A new coronavirus-like particle associated with diarrhea in swine. Arch Virol 58(3):243–247. https://doi.org/10.1007/bf01317606
Wang E, Guo D, Li C, Wei S, Wang Z, Liu Q et al (2016) Molecular characterization of the ORF3 and S1 genes of porcine epidemic diarrhea virus non S-INDEL strains in seven regions of China, 2015. Plos One 11(8):e0160561. https://doi.org/10.1371/journal.pone.0160561
Kamada N, Chen G, Inohara N, Núñez G (2013) Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 14(7):685–690. https://doi.org/10.1038/ni.2608
Wu T, Lyu Y, Li X, Wu M, Yu K, Li S et al (2020) Impact of N-acetylcysteine on the gut microbiota in the piglets infected with porcine epidemic diarrhea virus. Front Vet Sc 7:582338. https://doi.org/10.3389/fvets.2020.582338
Lindberg JE (2014) Fiber effects in nutrition and gut health in pigs. J Anim Sci Biotechnol 5(1):15. https://doi.org/10.1186/2049-1891-5-15
Yang H, Xiao Y, Wang J, Xiang Y, Gong Y, Wen X et al (2018) Core gut microbiota in Jinhua pigs and its correlation with strain, farm and weaning age. J Microbiol 56(5):346–55. https://doi.org/10.1007/s12275-018-7486-8
Cai R, Cheng C, Chen J, Xu X, Ding C, Gu B (2020) Interactions of commensal and pathogenic microorganisms with the mucus layer in the colon. Gut Microbes 11(4):680–690. https://doi.org/10.1080/19490976.2020.1735606
Abt M, Osborne L, Monticelli L, Doering T, Alenghat T, Sonnenberg G et al (2012) Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity 37(1):158–170. https://doi.org/10.1016/j.immuni.2012.04.011
Pfeiffer JK, Virgin HW (2016) Transkingdom control of viral infection and immunity in the mammalian intestine. Science 351(6270):aad5872. https://doi.org/10.1126/science.aad5872
Hergott C, Roche A, Tamashiro E, Clarke T, Bailey A, Laughlin A et al (2016) Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Am J Hematol 127(20):2460–2471. https://doi.org/10.1182/blood-2015-10-675173
Honda K, Littman DJN (2016) The microbiota in adaptive immune homeostasis and disease. Nature 535(7610):75–84. https://doi.org/10.1038/nature18848
Thaiss C, Zmora N, Levy M, Elinav EJN (2016) The microbiome and innate immunity. Nature 535(7610):65–74. https://doi.org/10.1038/nature18847
Tlaskalová-Hogenová H, Stepánková R, Hudcovic T, Tucková L, Cukrowska B, Lodinová-Zádníková R et al (2004) Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett 93(2–3):97–108. https://doi.org/10.1016/j.imlet.2004.02.005
Ma J, Piao X, Mahfuz S, Long S, Wang J (2022) The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Animal nutrition (Zhongguo xu mu shou yi xue hui) 9:159–174. https://doi.org/10.1016/j.aninu.2021.09.012
Priyadarshini M, Kotlo KU, Dudeja PK, Layden BT (2018) Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr Physiol 8(3):1091–1115. https://doi.org/10.1002/cphy.c170050
Ma X, Fan PX, Li LS, Qiao SY, Zhang GL, Li DF (2012) Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci 90(Suppl 4):266–268. https://doi.org/10.2527/jas.50965
Ferronato G, Prandini A (2020) Dietary supplementation of inorganic, organic, and fatty acids in pig: a review. Animals : an open access journal from MDPI 10(10). https://doi.org/10.3390/ani10101740
Diao H, Jiao AR, Yu B, Mao XB, Chen DW (2019) Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes Nutr 14:4. https://doi.org/10.1186/s12263-019-0626-x
Saleri R, Borghetti P, Ravanetti F, Cavalli V, Ferrari L, De Angelis E et al (2022) Effects of different short-chain fatty acids (SCFA) on gene expression of proteins involved in barrier function in IPEC-J2. Porc Health Manag 8(1):21. https://doi.org/10.1186/s40813-022-00264-z
Louis P, Hold G, Flint HJ (2014) The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 12(10):661–672. https://doi.org/10.1038/nrmicro3344
Rothhammer V, Mascanfroni I, Bunse L, Takenaka M, Kenison J, Mayo L et al (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22(6):586–597. https://doi.org/10.1038/nm.4106
Ivanov I, Littman DR (2011) Modulation of immune homeostasis by commensal bacteria 14(1):106–114. https://doi.org/10.1016/j.mib.2010.12.003
Song D, Park B (2012) Porcine epidemic diarrhoea virus: a comprehensive review of molecular epidemiology, diagnosis, and vaccines. Virus Genes 44(2):167–175. https://doi.org/10.1007/s11262-012-0713-1
Mole BJN (2013) Deadly pig virus slips through US borders. Nature 499(7459):388. https://doi.org/10.1038/499388a
Song D, Peng Q, Chen Y, Zhou X, Zhang F, Li A et al (2017) Altered gut microbiota profiles in sows and neonatal piglets associated with porcine epidemic diarrhea virus infection. Sci Rep 7(1):17439. https://doi.org/10.1038/s41598-017-17830-z
Huang M, Wang S, Wang H, Cui D, Yang Y, Liu X et al (2018) Differences in the intestinal microbiota between uninfected piglets and piglets infected with porcine epidemic diarrhea virus. Plos One 13(2):e019299210. https://doi.org/10.1371/journal.pone.0192992
Tan Z, Dong W, Ding Y, Ding X, Zhang Q, Jiang LJG (2019) Porcine epidemic diarrhea altered colonic microbiota communities in suckling piglets 11(1). https://doi.org/10.3390/genes11010044
Tan Z, Dong W, Ding Y, Ding X, Zhang Q, Jiang LJG (2019) Changes in cecal microbiota community of suckling piglets infected with porcine epidemic diarrhea virus. Plos One 14(7)
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165(6):1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Cummings J, Pomare E, Branch W, Naylor C, Macfarlane GJG (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28(10):1221–1227. https://doi.org/10.1136/gut.28.10.1221
Fernandes J, Su W, Rahat-Rozenbloom S, Wolever T, Comelli EJN (2014) Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 4(6)
Vogt S, Peña-Díaz J, Finlay BJA (2015) Chemical communication in the gut: effects of microbiota-generated metabolites on gastrointestinal bacterial pathogens 34:106–115. https://doi.org/10.1016/j.anaerobe.2015.05.002
Canfora E, Jocken J, Blaak EE (2015) Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11(10):577–591. https://doi.org/10.1038/nrendo.2015.128
Sun M, Wu W, Liu Z, Cong Y (2017) Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52(1):1–8. https://doi.org/10.1007/s00535-016-1242-9
Bloemen J, Venema K, van de Poll M, Olde Damink S, Buurman W, Dejong CH (2009) Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr 28(6):657–661. https://doi.org/10.1016/j.clnu.2009.05.011
Lin M, de Zoete M, van Putten J, Strijbis K (2015) Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front Immunol 6:554. https://doi.org/10.3389/fimmu.2015.00554
Vinolo M, Hatanaka E, Lambertucci R, Newsholme P, Curi R (2009) Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct 27(1):48–55. https://doi.org/10.1002/cbf.1533
Vinolo M, Rodrigues H, Hatanaka E, Sato F, Sampaio S, Curi R (2011) Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem 22(9):849–855. https://doi.org/10.1016/j.jnutbio.2010.07.009
Furusawa Y, Obata Y, Fukuda S, Endo T, Nakato G, Takahashi D et al (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504(7480):446–450. https://doi.org/10.1038/nature12721
Haghikia A, Jörg S, Duscha A, Berg J, Manzel A, Waschbisch A et al (2016) Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 44(4):951–953. https://doi.org/10.1016/j.immuni.2016.04.006
Dong W, Ding N, Zhang Y, Tan Z, Ding X, Zhang Q et al (2021) Lactobacillus salivarius alterations of suckling piglet jejunal microbiota due to infection with porcine epidemic diarrhea virus and protection against infection by. Front Vet 8:771411. https://doi.org/10.3389/fvets.2021.771411
Madson D, Magstadt D, Arruda P, Hoang H, Sun D, Bower L et al (2014) Pathogenesis of porcine epidemic diarrhea virus isolate (US/Iowa/18984/2013) in 3-week-old weaned pigs 174:60–8. https://doi.org/10.1016/j.vetmic.2014.09.002
Jung K, Wang Q, Scheuer KA, Lu Z, Zhang Y, Saif LJ (2014) Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg Infect Dis 20(4):662. https://doi.org/10.3201/eid2004.131685
Gurav A, Sivaprakasam S, Bhutia YD, Boettger T, Singh N, Ganapathy V (2015) Slc5a8, a Na+-coupled high-affinity transporter for short-chain fatty acids, is a conditional tumour suppressor in colon that protects against colitis and colon cancer under low-fibre dietary conditions 469(2):267–78. https://doi.org/10.1042/bj20150242
Miyauchi S, Gopal E, Fei YJ, Ganapathy V (2004) Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids 279(14):13293–6. https://doi.org/10.1074/jbc.C400059200
Halestrap A, Wang X, Poole R, Jackson V, Price NT (1997) Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol 80:17A-25A. https://doi.org/10.1016/s0002-9149(97)00454-2
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH (2013) Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice 145(2):396–406.e1–10. https://doi.org/10.1053/j.gastro.2013.04.056
Ragsdale SW, Pierce E (2008) Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation 1784(12):1873–98. https://doi.org/10.1016/j.bbapap.2008.08.012
Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C (2012) Bacteria and host interactions in the gut epithelial barrier. Nat Chem Biol 8(1):36–45. https://doi.org/10.1038/nchembio.741
Yan H, Ajuwon KM (2017) Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One 12(6):e0179586. https://doi.org/10.1371/journal.pone.0179586
Brosnahan AJ, Brown DR (2012) Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol 156(3–4):229–237. https://doi.org/10.1016/j.vetmic.2011.10.017
Manning BD, Cantley LC (2007) AKT/PKB signaling: navigating downstream. Cell 129(7):1261–1274. https://doi.org/10.1016/j.cell.2007.06.009
Zhao X, Jiang L, Fang X, Guo Z, Wang X, Shi B et al (2022) Host-microbiota interaction-mediated resistance to inflammatory bowel disease in pigs. Microbiome 10(1):115. https://doi.org/10.1186/s40168-022-01303-1
Funding
This work was supported by the Science and Technology Development Program of Jilin Province (YDZJ202301ZYTS326), National Natural Science Foundation of China (U21A20261, 32202819, 31941018, 31972696 and 32072888), the China Agriculture Research System of MOF and MARA (CARS-35), the Science and Technology Development Program of Jilin Province (20190301042NY, YDZJ202102CXJD029, and 20210202102NC).
Author information
Authors and Affiliations
Contributions
C.-F.W. and C.-W.S. designed the experiments. M.-J.S. wrote the paper. Q.-S.Y. organized and typeset the images. T.-M.N., B.-S.Z., and Y.-J.W. helped with sample collection and data presentation. J.-H.X., T.Y., B.-S.Z., R.-N.Z, and Q.-S.Y. performed the majority of the experiments and analyzed the data. D.Z., T.-M.N., W.-S.S., S.-M.Z., and H.-B.H. helped revise the manuscript W.-S.S., and S.-M.Z. supervised the study. M.-S.Z. and D.Z. drafted the original paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, MJ., Xing, J.H., Yan, QS. et al. The Acetic Acid Produced by Lactobacillus Species Regulates Immune Function to Alleviate PEDV Infection in Piglets. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10243-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10243-1