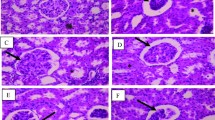
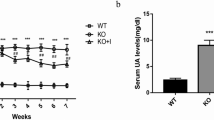
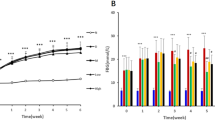
Abstract
Dairy foods have become an interest in chronic kidney disease (CKD) due to their nutritional profile, which makes them a good substrate for probiotics incorporation. This study evaluated the effect of probiotic-enriched Minas cheese with Lactobacillus acidophilus La-05 in an experimental rat model for CKD on cardiac, inflammatory, and oxidative stress parameters. Male Wistar rats were divided into 4 groups (n = 7/group): 5/6 nephrectomy + conventional Minas cheese (NxC); 5/6 nephrectomy + probiotic Minas cheese (NxPC); Sham + conventional Minas cheese (ShamC); Sham + probiotic Minas cheese (ShamPC). Offering 20 g/day of Minas cheese with Lact. acidophilus La-05 (108–109 log CFU/g) for 6 weeks. The cardiomyocyte diameter was determined. Superoxide dismutase (SOD) activity in plasma, heart, kidney, and colon tissue was performed. At the end of supplementation, no significant changes in lipid profile and renal parameters were found. The NxPC group showed a decrease in cardiomyocyte diameter compared to the NxC group (16.99 ± 0.85 vs. 19.05 ± 0.56 μm, p = 0.0162); also they showed reduced plasmatic SOD activity (502.8 ± 49.12 vs. 599.4 ± 94.69 U/mL, p < 0.0001). In summary, probiotic-enriched Minas cheese (Lact. acidophilus La-05) consumption suggests a promisor cardioprotective effect and was able to downregulate SOD activity in a rat model of CKD.
Graphical Abstract







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author, Silva Costa N and Araujo JR, upon reasonable request.
References
Wittwer AE, Lee SG, Ranadheera CS (2023) Potential associations between organic dairy products, gut microbiome, and gut health: A review. Food Res Int 172:113195. https://doi.org/10.1016/j.foodres.2023.113195
Aslam H, Marx W, Rocks T, Loughman A, Chandrasekaran V, Ruusunen A, Dawson SL, West M, Mullarkey E et al (2020) The effects of dairy and dairy derivatives on the gut microbiota: a systematic literature review. Gut Microbes 12(1):1799533. https://doi.org/10.1080/19490976.2020.1799533
Şanlier N, Gökcen BB, Sezgin AC (2019) Health benefits of fermented foods. Crit Rev Food Sci Nutr 59:506–527. https://doi.org/10.1080/10408398.2017.1383355
Abdul Hakim BN, Xuan NJ, Oslan SNH (2023) A comprehensive review of bioactive compounds from lactic acid bacteria: Potential functions as functional food in dietetics and the food industry. Foods 12(15):2850. https://doi.org/10.3390/foods12152850
Rocha RS, Silva R, Guimarães JT et al (2020) Possibilities for using ohmic heating in minas frescal cheese production. Food Res Int 131:109027. https://doi.org/10.1016/j.foodres.2020.109027
Scudino H, Guimarães JT, Silva Moura R et al (2023) Thermosonication as a pretreatment of raw milk for Minas frescal cheese production. Ultrason Sonochem 92:106260. https://doi.org/10.1016/j.ultsonch.2022.106260
Silva CB, Ferreira LM, Lima AR et al (2023) Microbiological quality and cultivable bacterial community of fresh and ripened Minas cheeses made from raw and pasteurised milk. Int Dairy J 143:105662. https://doi.org/10.1016/j.idairyj.2023.105662
Sperry MF, Silva HLA, Balthazar CF et al (2018) Probiotic Minas Frescal cheese added with L. casei 01: Physicochemical and bioactivity characterization and effects on hematological/biochemical parameters of hypertensive overweighted women – A randomized double-blind pilot trial. J Funct Foods 45:435–443. https://doi.org/10.1016/j.jff.2018.04.015
Mafra D, Borges N, Alvarenga L et al (2019) Dietary components that may influence the disturbed gut microbiota in chronic kidney disease. Nutrients 11:496. https://doi.org/10.3390/nu11030496
Podkowińska A, Formanowicz D (2020) Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants (Basel) 9:752. https://doi.org/10.3390/antiox9080752
Merino-Ribas A, Araujo R, Pereira L et al (2022) Vascular calcification and the gut and blood microbiome in chronic kidney disease patients on peritoneal dialysis: A pilot study. Biomolecules 12:867. https://doi.org/10.3390/biom12070867
Lekawanvijit S (2018) Cardiotoxicity of uremic toxins: A driver of cardiorenal syndrome. Toxins (Basel) 10:352. https://doi.org/10.3390/toxins10090352
Kamath N, Iyengar AA (2017) Chronic kidney disease (CKD): An observational study of etiology, severity and burden of comorbidities. Indian J Pediatr 84:822–825. https://doi.org/10.1007/s12098-017-2413-2
Zhu H, Cao C, Wu Z et al (2021) The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell Metab 33:1926–1942.e8. https://doi.org/10.1016/j.cmet.2021.06.014
Natarajan R, Pechenyak B, Vyas U et al (2014) Randomized controlled trial of strain-specific probiotic formulation (renadyl) in dialysis patients. Biomed Res Int 2014:568571. https://doi.org/10.1155/2014/568571
Alatriste PVM, Arronte RU, Espinosa COG, Cuevas MDLÁ (2014) Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp 29:582–590. https://doi.org/10.3305/nh.2014.29.3.7179
Kooshki A, Tofighiyan T, Miri M (2019) A synbiotic supplement for inflammation and oxidative stress and lipid abnormalities in hemodialysis patients. Hemodial Int 23:254–260. https://doi.org/10.1111/hdi.12748
Mirzaeian S, Saraf-Bank S, Entezari MH et al (2020) Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: A double-blind, placebo-controlled, randomized clinical trial. Nutrition 73:110713. https://doi.org/10.1016/j.nut.2019.110713
Borges NA, Carmo FL, Stockler-Pinto MB et al (2018) Probiotic supplementation in chronic kidney disease: A double-blind, randomized, placebo-controlled trial. J Ren Nutr 28:28–36. https://doi.org/10.1053/j.jrn.2017.06.010
Borges NA, Stenvinkel P, Bergman P et al (2019) Effects of probiotic supplementation on trimethylamine-n-oxide plasma levels in hemodialysis patients: A pilot study. Probiotics Antimicrob Proteins 11:648–654. https://doi.org/10.1007/s12602-018-9411-1
Vaziri ND, Wong J, Pahl M et al (2013) Chronic kidney disease alters intestinal microbial flora. Kidney Int 83(2):308–15. https://doi.org/10.1038/ki.2012.345
De Mauri A, Carrera D, Bagnati M et al (2022) Probiotics-supplemented low-protein diet for microbiota modulation in patients with advanced chronic kidney disease (ProLowCKD): Results from a placebo-controlled randomized trial. Nutrients 14(8):1637. https://doi.org/10.3390/nu14081637
Matijašić BB, Obermajer T, Lipoglavšek L et al (2016) Effects of synbiotic fermented milk containing Lactobacillus acidophilus La-5 and Bifidobacterium animalis ssp. lactis BB-12 on the fecal microbiota of adults with irritable bowel syndrome: A randomized double-blind, placebo-controlled trial. J Dairy Sci 99(7):5008–5021. https://doi.org/10.3168/jds.2015-10743
Borshchev YY, Burovenko IY, Karaseva AB et al (2022) Probiotic therapy with lactobacillus acidophilus and bifidobacterium animalis subsp. lactis results in infarct size limitation in rats with obesity and chemically induced colitis. Microorganisms 10(11):2293. https://doi.org/10.3390/microorganisms10112293
Meng L, Li S, Liu G et al (2021) The nutrient requirements of Lactobacillus acidophilus LA-5 and their application to fermented milk. J Dairy Sci 104(1):138–150. https://doi.org/10.3168/jds.2020-18953
Mokhtar NM, Jaafar NM, Alfian E et al (2021) Clinical assessment and cytokines level in constipation-predominant irritable bowel syndrome participants treated with Lactobacillus-containing cultured milk drink. Acta Gastroenterol Belg 84(4):585–591. https://doi.org/10.51821/84.4.009
Moura CS, Lollo PCB, Morato PN et al (2016) Assessment of antioxidant activity, lipid profile, general biochemical and immune system responses of Wistar rats fed with dairy dessert containing Lactobacillus acidophilus La-5. Food Res Int 90:275–280. https://doi.org/10.1016/j.foodres.2016.10.042
Vale GC, Mota BIS, Ando-Suguimoto ES, Mayer MPA (2023) Lactobacilli probiotics modulate antibacterial response gene transcription of dendritic cells challenged with LPS. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-023-10043-z
Wang X, Chaudhry MA, Nie Y et al (2017) A mouse 5/6th nephrectomy model that induces experimental uremic cardiomyopathy. J Vis Exp 55825. https://doi.org/10.3791/55825
Lollo PCB, Cruz AG, Morato PN et al (2012) Probiotic cheese attenuates exercise-induced immune suppression in wistar rats. J Dairy Sci 95:3549–3558. https://doi.org/10.3168/jds.2011-5124
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Costa RGB, Junior AC, da Cruz AG et al (2018) Effect of partial replacement of sodium chloride with potassium chloride on the characteristics of Minas Padrão cheese. Int Dairy J. https://doi.org/10.1016/j.idairyj.2018.12.002
Aoac International Official Methods of AnalysisTM, 21st Edition (2019) Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/. Accessed 13 Dec 2022
Gomes AA, Braga SP, Cruz AG et al (2011) Effect of the inoculation level of lactobacillus acidophilus in probiotic cheese on the physicochemical features and sensory performance compared with commercial cheeses. J Dairy Sci 94:4777–4786. https://doi.org/10.3168/jds.2011-4175
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502. https://doi.org/10.1093/clinchem/18.6.499
Besseling PJ, Pieters TT, Nguyen ITN et al (2021) A plasma creatinine- and urea-based equation to estimate glomerular filtration rate in rats. Am J Physiol-Ren Physiol 320:F518–F524. https://doi.org/10.1152/ajprenal.00656.2020
Boldt K, Rios JL, Joumaa V, Herzog W (2021) Mechanical function of cardiac fibre bundles is partly protected by exercise in response to diet-induced obesity in rats. Appl Physiol Nutr Metab 46:46–54. https://doi.org/10.1139/apnm-2020-0275
Yin FC, Spurgeon HA, Rakusan K et al (1982) Use of tibial length to quantify cardiac hypertrophy: Application in the aging rat. Am J Physiol-Heart Circ Physiol 243:H941–H947. https://doi.org/10.1152/ajpheart.1982.243.6.H941
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Levine RL, Garland D, Oliver CN et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol; Elsevier, 186:464–478 ISBN 978-0-12-182087-9. https://doi.org/10.1016/0076-6879(90)86141-h
Almeida PP, de Moraes Thomasi BB, Menezes ÁC et al (2022) 5/6 nephrectomy affects enteric glial cells and promotes impaired antioxidant defense in the colonic neuromuscular layer. Life Sci 298:120494. https://doi.org/10.1016/j.lfs.2022.120494
Garbowska M, Pluta A, Berthold-Pluta A (2020) Contents of functionally bioactive peptides, free amino acids, and biogenic amines in dutch-type cheese models produced with different lactobacilli. Molecules 25:5465. https://doi.org/10.3390/molecules25225465
Stobiecka M, Król J, Brodziak A (2022) Antioxidant activity of milk and dairy products. Animals (Basel) 12:245. https://doi.org/10.3390/ani12030245
Kurbanova M, Voroshilin R, Kozlova O, Atuchin V (2022) Effect of lactobacteria on bioactive peptides and their sequence identification in mature cheese. Microorganisms 10:2068. https://doi.org/10.3390/microorganisms10102068
Wang H, Li L (2022) Comprehensive evaluation of probiotic property, hypoglycemic ability and antioxidant activity of lactic acid bacteria. Foods 11:1363. https://doi.org/10.3390/foods11091363
Bao YW, Yuan Y, Chen JH, Lin WQ (2018) Kidney disease models: Tools to identify mechanisms and potential therapeutic targets. Zool Res 39(2):72–86. https://doi.org/10.24272/j.issn.2095-8137.2017.055
Hanifa MA, Skott M, Maltesen RG et al (2020) Tissue, urine and serum NMR metabolomics dataset from a 5/6 nephrectomy rat model of chronic kidney disease. Data Brief 33:106567. https://doi.org/10.1016/j.dib.2020.106567
Almeida PP, Da Cruz BO, Thomasi B et al (2023) Brazil nut-enriched diet modulates enteric glial cells and gut microbiota in an experimental model of chronic kidney disease. J Am Nutr Assoc 1–12. https://doi.org/10.1080/27697061.2023.2247057
Huang H, Li K, Lee Y, Chen M (2021) Preventive effects of Lactobacillus Mixture against chronic kidney disease progression through enhancement of beneficial bacteria and downregulation of gut-derived uremic toxins. J Agric Food Chem 69:7353–7366. https://doi.org/10.1021/acs.jafc.1c01547
Martins AA, Santos-Junior VA, Filho ERT et al (2018) Probiotic prato cheese consumption attenuates development of renal calculi in animal model of urolithiasis. J Funct Foods 49:378–383. https://doi.org/10.1016/j.jff.2018.08.041
Maiuolo J, Oppedisano F, Gratteri S, Muscoli C, Mollace V (2016) Regulation of uric acid metabolism and excretion. Int J Cardiol 213:8–14. https://doi.org/10.1016/j.ijcard.2015.08.109
Kuo YW, Hsieh SH, Chen JF et al (2021) Lactobacillus reuteri tsr332 and lactobacillus fermentum TSF331 stabilize serum uric acid levels and prevent hyperuricemia in rats. PeerJ 9:e11209. https://doi.org/10.7717/peerj.11209
Yamada N, Saito-Iwamoto C, Nakamura M et al (2017) Lactobacillus gasseri PA-3 uses the purines IMP, inosine and hypoxanthine and reduces their absorption in rats. Microorganisms 5:10. https://doi.org/10.3390/microorganisms5010010
Vaziri ND, Zhao YY, Pahl MV (2016) Altered intestinal microbial flora and impaired epithelial barrier structure and function in CKD: The nature, mechanisms, consequences and potential treatment. Nephrol Dial Transpl 31:737–746. https://doi.org/10.1093/ndt/gfv095
Wong J, Piceno YM, DeSantis TZ et al (2014) Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol 39:230–237. https://doi.org/10.1159/000360010
Koppe L, Mafra D, Fouque D (2015) Probiotics and chronic kidney disease. Kidney Int 88:958–966. https://doi.org/10.1038/ki.2015.255
Vaziri ND, Yuan J, Norris K (2013) Role of urea in intestinal barrier dysfunction and disruption of epithelial tight junction in chronic kidney disease. Am J Nephrol 37:1–6. https://doi.org/10.1159/000345969
Di Lullo L, House A, Gorini A et al (2015) Chronic kidney disease and cardiovascular complications. Heart Fail Rev 20:259–272. https://doi.org/10.1007/s10741-014-9460-9
Lollo PCB, Morato PN, Moura CS et al (2015) Hypertension parameters are attenuated by the continuous consumption of probiotic minas cheese. Food Res Int 76:611–617. https://doi.org/10.1016/j.foodres.2015.07.015
Silva-Cutini MA, Almeida SA, Nascimento AM et al (2019) Long-term treatment with kefir probiotics ameliorates cardiac function in spontaneously hypertensive rats. J Nutr Biochem 66:79–85. https://doi.org/10.1016/j.jnutbio.2019.01.006
Klippel BF, Duemke LB, Leal MA et al (2016) Effects of kefir on the cardiac autonomic tones and baroreflex sensitivity in spontaneously hypertensive rats. Front Physiol 7:211. https://doi.org/10.3389/fphys.2016.00211
Huang CY, Nithiyanantham S, Liao JY, Lin W (2020) Bioactive peptides attenuate cardiac hypertrophy and fibrosis in spontaneously hypertensive rat hearts. J Food Drug Anal 28:94–102. https://doi.org/10.1016/j.jfda.2019.11.002
Falconi CA, Junho CVDC, Fogaça-Ruiz F et al (2021) Uremic toxins: An alarming danger concerning the cardiovascular system. Front Physiol 12:686249. https://doi.org/10.3389/fphys.2021.686249
Zhang Y, Wang S, Huang Y et al (2020) Inhibition of CYP1B1 ameliorates cardiac hypertrophy induced by uremic toxin. Mol Med Rep 21:393–404. https://doi.org/10.3892/mmr.2019.10810
Poulianiti KP, Kaltsatou A, Mitrou GI et al (2016) Systemic redox imbalance in chronic kidney disease: A systematic review. Oxid Med Cell Longev 2016:8598253. https://doi.org/10.1155/2016/8598253
Aranda-Rivera AK, Cruz-Gregorio A, Pedraza-Chaverri J, Scholze A (2022) Nrf2 activation in chronic kidney disease: Promises and pitfalls. Antioxidants 11:1112. https://doi.org/10.3390/antiox11061112
Zhang P, Han X, Zhang X, Zhu X (2021) Lactobacillus acidophilus ATCC 4356 alleviates renal ischemia–reperfusion injury through antioxidant stress and anti-inflammatory responses and improves intestinal microbial distribution. Front Nutr 8:667695. https://doi.org/10.3389/fnut.2021.667695
Rezazadeh L, Alipour B, Jafarabadi MA et al (2021) Daily consumption effects of probiotic yogurt containing lactobacillus acidophilus La5 and bifidobacterium lactis Bb12 on oxidative stress in metabolic syndrome patients. Clin Nutr ESPEN 41:136–142. https://doi.org/10.1016/j.clnesp.2020.12.003
Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A et al (2012) Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 28:539–543. https://doi.org/10.1016/j.nut.2011.08.013
Vida C, Oliva C, Yuste C et al (2021) Oxidative stress in patients with advanced CKD and renal replacement therapy: The key role of peripheral blood leukocytes. Antioxidants (Basel) 10:1155. https://doi.org/10.3390/antiox10071155
Mapuskar KA, Wen H, Holanda DG et al (2018) Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol 20:98–106. https://doi.org/10.1016/j.redox.2018.09.020
Nemmar A, Al-Salam S, Beegam S et al (2021) Cardiac inflammation, oxidative stress, Nrf2 expression, and coagulation events in mice with experimental chronic kidney disease. Oxid Med Cell Longev 8845607. https://doi.org/10.1155/2021/8845607
Mota JC, Almeida PP, Freitas MQ et al (2022) Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem 402:134351. https://doi.org/10.1016/j.foodchem.2022.134351
Tucker PS, Scanlan AT, Dalbo VJ (2015) Chronic kidney disease influences multiple systems: Describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid Med Cell Longev 2015:806358. https://doi.org/10.1155/2015/806358
Yin L, Li X, Ghosh S et al (2021) Role of gut microbiota-derived metabolites on vascular calcification in CKD. J Cell Mol Med 25:1332–1341. https://doi.org/10.1111/jcmm.16230
Huang L, Zhao Z, Duan C et al (2019) Lactobacillus plantarum C88 protects against aflatoxin B1-induced liver injury in mice via inhibition of NF-ΚB–mediated inflammatory responses and excessive apoptosis. BMC Microbiol 19:170. https://doi.org/10.1186/s12866-019-1525-4
Bron PA, Tomita S, Mercenier A, Kleerebezem M (2013) Cell surface-associated compounds of probiotic lactobacilli sustain the strain-specificity dogma. Curr Opin Microbiol 16:262–269. https://doi.org/10.1016/j.mib.2013.06.001
Acknowledgements
We are thankful to the following laboratories for their technical assistance: Experimental Nutrition Laboratory (LabNE)- Fluminense Federal University for allowing the use of animal houses, Unidade de Pesquisa Clínica (UPC) to assist in the use of equipment, and Multi-user Physiology and Pharmacology Laboratory (LAMFFA), including the use of ChemiDoc MP. The authors would also like to express their thanks to all the collaborators involved in the routine activities of the laboratories.
Funding
The Brazilian foundations supported this project and scholarships: The State of Rio de Janeiro Carlos Chagas Filho Research Foundation (FAPERJ) (Process E-26/201.373/2021), National Counsel of Technological and Scientific Development (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
Nathalia da Silva Costa: Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization; Joana Ramos de Araujo: Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization; Manuela Fernandes da Silva Melo: Formal analysis, Investigation, Writing – review & editing; Jéssica da Costa Mota: Formal analysis, Investigation, Writing – review & editing; Patrícia Pereira de Almeida: Formal analysis, Data curation, Writing – review & editing; Karen Salve Coutinho-Wolino: Investigation, Writing – review & editing; Beatriz Oliveira Da Cruz: Investigation, Data curation, Writing – review & editing; Michele Brito Lima: Investigation, Writing – review & editing; Thaís de Souza Carvalho: Investigation, Writing – review & editing; Emanuelle Barreto-Reis: Investigation, Writing – review & editing; Beatriz Gouvêa de Luca: Investigation, Writing – review & editing; Denise Mafra: Resources, Writing – review & editing; D’Angelo Carlo Magliano Resources, Writing – review & editing; Renato de Souza Abboud: Resources, Writing – review & editing; Ramon Silva Rocha: Resources, Writing – review & editing; Adriano Gomes da Cruz: Methodology, Resources, Writing – review & editing; Jonas de Toledo Guimarães: Methodology, Data curation, Writing – review & editing; Milena Barcza Stockler-Pinto: Conceptualization, Methodology, Resources, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva Costa, N., de Araujo, J.R., da Silva Melo, M.F. et al. Effects of Probiotic-Enriched Minas Cheese (Lactobacillus acidophilus La-05) on Cardiovascular Parameters in 5/6 Nephrectomized Rats. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10173-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10173-4