Abstract
More scientific study and methods that are compatible with the honeybee-specific probiotic bacteria are needed in modern beekeeping to increase the productivity and well-being of honeybees. The goal of the current study set out to investigate the possible effects of probiotics previously isolated from the honeybee intestinal tract and soybean patties on nurse worker bee hypopharyngeal gland (HPG) development. The experimentation was carried out in four different treatment groups in which probiotics and soybean patties were provided in different proportions, with control colonies. Results showed that there was a significant increase in HPG morphometric parameters of bees in all experimental groups. Control nurse worker fed with sugar syrup for only 2 weeks had the smallest HPG morphometric parameters. The highest HPG diameter 14.89 ± 0.097 µm and surface area 0.065 ± 0.001µm2 were observed in the bees group fed with both probiotic and soya patty. Additionally, the same trend was observed in all morphometric parameters with the bees group fed with probiotic bacteria and soya patty. More royal jelly can be produced by larger HPGs than by smaller ones. Thus, the use of probiotics as a natural alternative tool boosted the development of Apis mellifera nurse workers’ HPG that will positively affect the beekeepers’ economy by providing a higher yield of royal jelly production. Overall, the study’s findings show that probiotics are a useful feed supplement for honeybees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because they pollinate a wide range of crops that are used by both people and animals, honeybees are crucial to agriculture. However, the number of honeybee colonies worldwide has not kept up with the escalating demand [1]. Colony losses have worsened by several interacting biotic and abiotic factors [2, 3]. Nutrition is critical to the ability of an organism to survive difficulties and challenges throughout its lifecycle. Optimal nutrition throughout colony growth, either through access to diverse natural forage [4] or as nutritional supplements [5]. Healthy colony growth results from increased brood production which is dependent on the presence of a healthy population of nurse bees within the colony that produce appropriate brood food via glandular secretions [6]. Glands that produce food provisioned for brood development are the mandibular and hypopharyngeal glands (HPGs) [7, 8]. HPGs are made up of several acini grouped in coils and loops. The largest gland in the skull cavity is thought to be HPG. Each HPG has a thousand or more pear-shaped lobules linked to a long duct, and each lobule is further made up of numerous single-celled glands [9]. The size of HPGs has long been used as a reliable marker of honey bee nutritional status [10]. Additionally, royal action is a key component that facilitates the transformation of growing larvae into queens morphologically [11]. Because queens eat royal jelly, they live longer than normal bees. As a result, the growth of HPGs and the secretory activity of glands directly influence worker behavior, and the colony’s viability may also be impacted. Due to their ability to increase animal output and have growth-stimulating qualities, some phytogenic chemicals received additional attention in the recent past. Previous studies have shown that probiotics improved longevity, productivity, and pathogen tolerance [12, 13]. Here, we determine the HPG size in nurse bees supplemented with probiotic bacteria isolated from the bee’s intestinal tract [14, 15].
“Live microorganisms that, when administered in sufficient proportions, confer a health benefit to the host” is how probiotics are described by ISAPP [16]. Originally, probiotics were implemented to promote immunological function, lower blood cholesterol, and prevent cancer by enhancing gut health, immune response, and both animal and human health [17]. Among the known probiotic microorganisms, species of lactic acid bacteria (LAB) (such as Lactococcus, Lactobacillus, Streptococcus, and Enterococcus) and Bifidobacterium have a long history of safe use [18]. Most of the research on the microbes connected to honeybees has been done with the aim of identifying and isolating the helpful bacteria connected to these bees. While substantial research has been done on the impact of stingless bee honey and probiotic LAB isolated from honeybees on honeybee pathogens, little is known about the physiological effects of probiotic bacteria in addition to how they affect colony reproduction. It is worth noting that there has not been much research on how probiotic feed affects the morphometric characteristics of the hypopharyngeal gland. Therefore, the aim of this study was to investigate the effect of probiotic bacteria as an additive to the supplementary feed on the size of nurse worker bees’ HPGs and the amount of secreted royal jelly.
Materials and Methods
Ethical Statement
The current research did not need any specific permits. Nurse worker bees for the experiments were obtained from experimental apiary of Cairo University’s Faculty of agriculture, Giza, Egypt. Apis mellifera in Honeybee Research Garden is not a protected or endangered species.
Experimental Honeybee Colonies Setup
According to the procedure outlined by William et al. [19], workers of A. mellifera aged 0 to 24 h were gathered by putting the frames containing a large amount of sealed brood in an incubator for 24 h at 30 °C temperatures and 70% humidity. The newly emerging workers were then moved into plastic cages with the dimensions 11 cm × 6 cm × 6 cm [9]. In an incubator, a bowl with a steel mesh top was also added to regulate humidity and prevent the newly emerged bees from falling into the bowl. In a plastic syringe feeder with a 5-mL capacity and a 3-mm pore, the sugar syrup was given to caged bees. Similarly, soybean patties were prepared using the technique outlined by [9]. The cages containing the fifty newly emerged bees were transferred right away to an incubator that was kept at 30 °C and 70% humidity [20, 21]. In 2022, an experiment was conducted from March to June.
Probiotics Culturing, Dose Preparation, and Counting C.F.U.
Tables 1 and 2 summarize the characteristics and administration information of the probiotic bacterial species employed in the current study. All strains used were isolated from the honeybee intestinal tract and molecularly identified based on the 16S rRNA gene sequences [14, 15].
Probiotic lactic acid bacterial strains were cultivated in MRS medium at 37 °C in a 5% carbon dioxide prior to incubation for overnight (18 h). The inoculum count was adjusted at OD600 = 0.1 (107 CFU/mL) using a spectrophotometer. Subsequently, 1 mL of the MRS broth with 107C.F.U. and 0.1 O.D. was taken in an Eppendorf tube and it was centrifuged at 15,000 rpm for 10 min at 25–30 °C. Later on, the supernatant was discarded, and the pellet was maintained in 15% glycerol and stored at − 20 °C. According to Martin-Hernandez et al. [22], sugar syrup was made by mixing sucrose and distilled water (1:2). With light modifications of C.F.U. and vehicle in the experiment of Hassan et al. [9], in the current experiments, 1 × 107C.F.U. and sugar syrup were provided with probiotic bacteria pellets and the bees were fed on soya bean patty. Respective sugar syrups in 100-mL quantity and soya bean patty 100 g were provided to cage twice a week.
Nurse Worker Samples and Heads Dissection
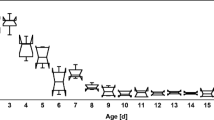
Samples of nursing bees at different ages, ranging from 1 to 15 days, were collected at the time of introduction into the colonies and then every 5 days after that. Five workers from each age and experimental group were sampled. The heads of the studied worker bees were fixed using two entomological needles on a rubber base (Xantopren® L blue and Activator universal, Heraeus Kulzer, Germany) in Hyes’ solution (NaCl 9.0 g, KCl 0.2 g, CaCl 0.2 g, NaHCO3 0.1 g, 1 l distilled water, pH 8.5) on a petri dish. For dissection, the stereomicroscope was employed (SterREO Discovery.V12, Zeiss). The external chitinous exoskeleton of the head’s facial region was removed between the compound eyes in order to collect the proper morphometric measurements of the glands.
Hypopharyngeal Glands Morphometric Parameters Using Image Analysis
The hypopharyngeal glands were photographed using a scanning electron microscope (SEM). To fix the protein, the HPG samples were fixed for 2 h in 3% glutaraldehyde in 0.1 M phosphate buffer at pH 7.2. In 15-min intervals, samples were washed numerous times in 0.1 M phosphate buffer (pH 7.2). They were then dehydrated for 5 min each in a series of aqueous ethanol solutions (25%, 50%, 75%, 95%, and 100%). The samples were placed on aluminum SEM stubs and sputter-coated with gold after being dried to critical point with CO2 in a critical point dryer (Polaron, Waterford, England) (SPI module sputter coater, SPI Supplies Division of Structure Probe, Inc). Samples were analyzed using a scanning electron microscope at 10 to 25 kV [14]. For image analysis, Scanning Prop Image Processor (SPIP) program 8 (BETA, Denmark) was used which enables the user to manipulate lateral calibration and unit cell detection to account for magnification differences in each image.
After calibration of the program according to the scale bar on the micrographs, HPG images were magnified for better definition. Detection and quantification of HPGs were done using the polygon measure shape. The program was keeping the measurements in memory and calculating some statistical values. Several morphological and geometrical parameters such as diameter, area, length, breadth, perimeter, and roundness were calculated by the system.
Measuring of Royal Jelly Yield
According to the method described by Khan and Ghramh [23], a microspatula was used to collect RJ from the cells into a plastic container, and its weight was calculated with an electronic scale (AL204-IC, Mettler Toledo, Switzerland). RJ was collected and stored at − 20 °C in the fridge.
Statistical Analysis
Mean ± standard deviation (SD) values are used to express the data. A randomize complete block design and analysis of variance of factorial methods were done using Mstat 4.0 statistical software (Norman Drinkwater, McArdle Laboratory). All data were analyzed in three replications for each parameter. The least significant differences (LSD) test was applied to compare the significant differences between the mean values for different treatments. At p < 0.05, the results were found to be statistically significant.
Results
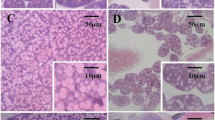
Results showed a favorable effect of probiotic feed supplementation on the development of HPGs. The glands were photographed, and representative images are shown in Fig. 1. Commonly, different scales are used in vertical and horizontal axes. It is typical, when using SEM, to observe a 3-dimensional spherical object such as glands that the central region of the image is darker than the periphery. SEM observations revealed that the HPG took the shape of long clusters surrounding an elongated central axial duct. The glands were paired structures composed of numerous secretory units, or acini that were connected to the central axial duct by thin, individual, excretory canaliculi. As shown in Fig. 1, larger acini size has come from feeding the bees with probiotic bacteria and soya patty as a source for protein compared to the control group which fed on sugar syrup. Because the hypopharyngeal gland size is sensitive to the amount of protein and pollen in the diet and is a critical marker of nourishment in bees, the greatest acinal area of HGs was observed when honeybees fed on probiotic bacteria and soya patty mixture (2:1) followed by probiotic bacteria and soya patty mixture (1:1), while honeybees fed on sucrose solution had a lower size of HGs compared to those fed on probiotic bacteria.
Control bees which were fed with sugar syrup for 2 weeks depicted the mean value of 13.07 ± 0.158 µm for diameter while bees of treatment (4) were fed with probiotic bacteria and soya bean patty recorded 14.89 ± 0.097 µm for diameter. Likewise, worker bees of treatment (1) and treatment (4) showed mean values of surface area as 0.047 ± 0.001 and 0.065 ± 0.001 µm2, respectively. Statistically significant differences (LSD = 0.1458, 0.1031) were found within HPG diameters and surface area means as a function of feeding formula (α = 0.05). The feeding treatments affected the HPG diameter and surface area significantly (P < 0.001) as shown in Table 3. Roundness was measured through the two-dimension image analysis. The maximum roundness for the tested HPGs was 0.604 ± 0.60 for treatment (4) and the minimum was 0.431 ± 0.06 for the control.
Roundness of HPGs showed more samples far away from being a perfect circle as found for treatment (2) and treatment (3) (0.523 ± 0.15, 0.548 ± 0.25), respectively, which indicated irregular circles (Fig. 1). It was worth mentioning that it was impossible to measure the length of many HPGs due to their curved shape. Additional1y, the glands’ edges were, in many cases, difficult to measure. For that gland, images with a straight and clear visibility were measured. The maximum and minimum length values of are presented in Table 3. Great differences (P < 0.001) were found in the length of the hypopharyngeal glands. In addition, wide perimeter ranges from 4.02 μm for the control to 4.74 μm for bees fed with probiotic bacteria and soya bean patty. Significant influence (P < 0.05) for perimeters was found as a function of type of feeding (LSD = 0.5834 at α = 0.05). By comparing data of the hypopharyngeal gland image, it becomes evident that there is a significant difference within width of the glands.
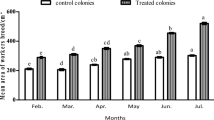
The average weight of royal jelly (RJ) per cup (mg) between different treatments compared to the control is mentioned, respectively (Fig. 2). The RJ yield was statistically greater in bees fed on probiotic bacteria mixed with soya patty EX3 and Ex4, respectively, in comparison to control bee colonies (p < 0.001). The maximum RJ yield was 219.23 ± 0.51 mg in bees fed with probiotic bacteria and soya patty (2:1) (Ex4). Similarly for bees fed on probiotic bacteria 50% and soya patty 50% (Ex3), the RJ yield was 199.273 ± 0.76 mg, while in the bees fed with soya patty the RJ yield was 184.57 ± 0.33 mg and the bees fed on probiotic bacteria the RJ yield was 184.11 ± 0.23 mg. The amount of RJ yield does not show statistically significant differences among both bees fed either probiotic bacteria only or soya bean patty diet.
Discussion
Understanding the morphogenesis of honeybee HPGs and the factors which regulate HPG development is essential for further investigations of the functionality of the glands [24]. HPGs are age-dependent structures in honeybees that change with the acinus size and correlate with different social behavior, especially, the quantity and quality of food as mentioned by [25, 26]. Nurse hypopharyngeal glands produce the protein fraction of the worker and royal jelly that is fed to developing larvae and queens [27]. The glands get smaller when nurses are fed deficient diets and are large when they are fed complete diets. Mao et al. [27] mentioned that nurse hypopharyngeal gland size is a robust indicator of nurse nutrition and health which can be improved with the use of probiotics. We noticed an increase in HPG size and in bees that were fed with probiotics. The results can be explained by the fact that the physiological status of bees reacted positively with the presence of beneficial microbes in the food as reported by [28, 29]. Feeding bee colonies on sugar syrup incorporating soya bean led to the development of glands greater in diameter than those of bees in the control group, likewise, in surface area, perimeter, length, and roundness. Bees given probiotic supplement in their feed developed hypopharyngeal gland greater in morphometric parameters than those in the control group, while supplementation of feed with both soya bean patty as a source for protein and a probiotic bacteria led to a better development of glands than those found in bees fed pure sugar syrup or soya patty. Mentioned results of HPG morphometric parameter agreed with some authors as [9, 30,31,32]. This greater development of glands as a result of feeding bees with probiotic is correlated with increased gland size resulting in increased royal jelly production. Our results agree with those of [33,34,35].
Royal jelly yield increased by increasing the HPG size resulting from probiotic feed. This greater development of HPG cells as a result of feeding bees with probiotic and soya bean patty is correlated with increased glandular activity resulting in increased RG production. However, more research is required to better understand how different probiotic strains affect the RJ yields.
Conclusions
It is worth noting that incorporation of probiotics in honeybee’s feed proves good for pollinator’s overall health. This study helps to obtain higher royal jelly yields. Future studies on the assessment of various other probiotic blends at pilot and commercial scales are needed for obtaining exact quantitative results at higher scales.
Availability of Data and Material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hajalizadeh Z, Dayani O, Khezri A et al (2019) The effect of adding fennel (Foeniculum vulgare) seed powder to the diet of fattening lambs on performance, carcass characteristics and liver enzymes. Small Rumin Res 175:72–77. https://doi.org/10.1016/j.smallrumres.2019.04.011
Neumann P, Blacquiere T (2017) The Darwin in cure for apiculture? Natural selection and managed honeybee health. Evolutionary Appl 10:226–230
van Engelsdorp D, Caron D, Hayes J, Underwood R, Hensen M, Rennich K, Spleen A, Andree M, Snyder R, Lee K, Roccasecca K, Wilson M, Wilkes J, Lengerich E, Pettis J (2012) A national survey of managed honey bee 2010–11 winter colony losses in the USA: results from the Bee Informed Partnership. J Apic Resh 51(1):115–124
Smart M, Otto C, Cornman R, Iwanowicz D (2018) Using colony monitoring devices to evaluate the impacts of land use and nutritional value of forage on honeybee health. Agriculture 8:2
Fedoriak M, Kulmanov O, Zhuk A, Shkrobanets O, Tymchuk K, Moskalyk G, Olendr T, Yamelynets T, Angelstam P (2021) Stakeholders’ views on sustaining honeybee health and beekeeping: the roles of ecological and social system drivers. Landsc Ecol 36:763–783
Doke MA, Frazier M, Grozinger CM (2015) Over wintering honeybees: biology and management. Curr Opin Insect Sci 10:185–193
Wang Y, Li Byarlay H (2015) Chapter two-physiological and molecular mechanisms of nutrition in honeybees. In: Jurenka R (ed) Adv Insect Physiol Academic Press, pp 25–58
Shakeel M, Ahmad S, Ali H et al (2020) Seasonal impact and comparative analysis of hypopharyngeal glands in worker and forager honey bees of two different species: Apis mellifera and A. cerana. Fresenius Environ Bull 29(10):9024–9030
Hasan A, Qazi JI, Tabssum F et al (2022) Feeding probiotics and organic acids to honeybees enhances acinal surface area of their hypopharyngeal glands. Res Vet Sci 149:47–50
De Grandi-Hoffman G, Chen Y, Huang E, Huang MH (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honeybees (Apis mellifera L.). J Insect Physiol 56:1184–1191
Kamakura M (2011) Royal actin induces queen differentiation in honeybees. Nature 473:478–483
Bernklau E, Bjostad L, Hogeboom A, Carlisle A, Arathi HS (2019) Dietary phytochemicals, honeybee longevity and pathogen tolerance. Insects 10:14
Geldert C, Abdo Z, Stewart JE, Arathi HS (2021) Dietary supplementation with phytochemicals improves diversity and abundance of honeybee gut microbiota. J Appl Microbiol 130:1705–1720
Elzeini HM, Ali AA, Nasr NF et al (2021) Isolation and identification of lactic acid bacteria from intestinal tract of honey bee Apis mellifera L. in Egypt. J Apic Res 60(2):349–357
Elzeini HM, Ali AA, Nasr NF et al (2021) Probiotic capability of novel lactic acid bacteria isolated from worker honeybee gut microbiota. FEMS Microbiol Lett 368(6):fnab030. https://doi.org/10.1093/femsle/fnab030
ISAPP (2018) Minimum Criteria for Probiotics [Online]. Sacramento; CA: International Scientific Association for Probiotics and Prebiotics
Kechagia M, Basoulis D, Konstantopoulou S et al (2013) Health benefits of probiotics: a review. ISRN Nutr. 2:481651. https://doi.org/10.5402/2013/481651. PMID: 24959545; PMCID: PMC4045285
Doron S, Snydman R (2015) Risk and safety of probiotics. Clin Infec Dise 60(2):129–134
William RJ, Alaux C, Costa C et al (2013) Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J Apic Res 1:52. https://doi.org/10.3896/IBRA.1.52.1.04
Evans JD, Chen YP, Prisco GD et al (2009) Bee cups: single-use cages for honeybee experiments. J Apic Res 48:300–302
Martín-Hernández R, Meana A, García-palencia P et al (2009) Effect of temperature on the biotic potential of honeybee microsporidia. Appl Environ Microbiol 75:2554–2557
Martín-Hernández R, Botías C, Barrios L (2011) Comparison of the energetic stress associated with experimental Nosema ceranae and Nosema apis infection of honeybees (Apis mellifera). Parasitol Res 109:605–612. https://doi.org/10.1007/s00436-011-2292-9
Khan KA, Ghramh HA (2022) Evaluation of queen cell acceptance and royal jelly production between hygienic and non-hygienic honey bee (Apis mellifera) colonies. PLoS ONE 17(3):e0266145. https://doi.org/10.1371/journal.pone.0266145
Klose SP, Rolke D, Baumann O (2017) Morphogenesis of honeybee hypopharyngeal gland during pupal development. Frontiers Zool 14:22
Cao LF, Zheng HQ, Pirk CW et al (2016) High royal jelly-producing honeybees (Apis mellifera ligustica) (Hymenoptera: Apidae) in China. J Econ Entomol 109:510–514
Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet J-L, Alaux C (2013) Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8:e72016
Mao W, Schuler MA, Berenbaum MR (2015) A dietary phytochemical alters caste associated gene expression in honeybees. Sci Adv 1
Corby-Harris V, Snyder L, Meador C, Ayotte T (2018) Honeybee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS ONE 13(1):e0191050
Ahmad S, Khan SA, Khan KA et al (2021) Novel insight into the development and function of hypopharyngeal glands in honey bees. Front Physiol 11:615830. https://doi.org/10.3389/fphys.2020.615830
Schneider P, Drescher W (1987) Varroa jacobsoni oud. Auf das schlupfgewicht, Die gewichtsentwicklung, die entwicklung Der hypopharynxdrüsen und die lebensdauer Von Apis mellifera L. Apidologie 18:101–110
Yousef S, El Basheir ZM, Teleb SS et al (2014) Effect of varroa infestation on the morphological and histological structure of the hypopharyngeal glands of Apis mellifera workers. Am J Sci 10(12):69–78
Gajger TI, Vlainić J, Šoštarić P et al (2020) Effects on some therapeutical, biochemical, and immunological parameters of honey bee (Apis mellifera) exposed to probiotic treatments, in field and laboratory conditions. Insects 11:638. https://doi.org/10.3390/insects11090638
Maes PW, Rodrigues PAP, Oliver R, Mott BM, Anderson KE (2016) Diet-related gut bacterial dysbiosis correlates with impaired development, increased mortality and Nosema disease in the honeybee (Apis mellifera). Mol Ecol 25:5439–5450
Ohashi K, Sasaki M, Sasagawa H et al (2000) Functional flexibility of the honey bee hypopharyngeal gland in a dequeened colony. Zool Sci 17:1089–1094
DeGrandi-Hoffman G, Chen Y, Huang E, Huang MH (2010) The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). J Insect Physiol 56(9):1184–1191
Acknowledgements
The authors are thankful to the Faculty of Agriculture, Cairo University, Giza, Egypt, for supporting by analytical samples at Cairo University Research Park (CURP) as well as Dairy science department lab.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AH and YE. The first draft of the manuscript was written by AH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, A.AM., Elenany, Y.E. Influence of Probiotics Feed Supplementation on Hypopharyngeal Glands Morphometric Measurements of Honeybee Workers Apis mellifera L.. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10107-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10107-0