Abstract
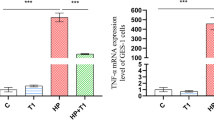
Capsaicin (CAP) is usually reported to have many biological activities. However, a large intake of CAP may cause heartburn, gastrointestinal pain, and diarrhea. In this study, mice were gavaged with nine lactic acid bacteria (LAB) strains for two weeks, in which the mice were treated with CAP at the second week and lasted for one week. We tried to identify potential probiotics that could prevent CAP-induced intestinal injury and investigate the mechanisms. The modulation of transient receptor potential vanilloid 1 (TRPV1), levels of short-chain fatty acids (SCFAs), and the composition of gut microbiota were analyzed. The results showed that Lactobacillus reuteri CCFM1175 and Lactobacillus paracasei CCFM1176 effectively attenuated CAP-induced injuries to the ileum and colon, including relieving the damage to colonic crypt structures, increasing the number of goblet cells, decreasing levels of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), increasing levels of anti-inflammatory factors (IL-10), and reducing levels of substance P (SP) and calcitonin gene-related peptide (CGRP) in serum and colon tissue. Further analysis showed that L. reuteri CCFM1175 increased the relative abundance of Ruminococcaceae UCG_014 and Akkermansia. L. paracasei CCFM1176 downregulated the expression of TRPV1 in the ileal and colonic tissues and promoted the relative abundance of Ruminococcaceae UCG_014 and Lachnospiraceae UCG_006. These results indicate that L. reuteri CCFM1175 and L. paracasei CCFM1176 could prevent CAP-induced intestinal injury and be used as probiotics to improve the gastrointestinal health.










Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Xiang Q, Guo W, Tang X, Cui S, Zhang F, Liu X, Zhao J, Zhang H, Mao B, Chen W (2021) Capsaicin-the spicy ingredient of chili peppers: a review of the gastrointestinal effects and mechanisms. Trends Food Sci Technol 116:755–765. https://doi.org/10.1016/j.tifs.2021.08.034
Srinivasan K (2016) Biological activities of red pepper (Capsicum annuum) and its pungent principle capsaicin: a review. Crit Rev Food Sci Nutr 56:1488–1500. https://doi.org/10.1080/10408398.2013.772090
van Avesaat M, Troost FJ, Westerterp-Plantenga MS, Helyes Z, Le Roux CW, Dekker J, Masclee AAM, Keszthelyi D (2016) Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am J Clin Nutr 103:305–313. https://doi.org/10.3945/ajcn.115.123414
Hammer J, Vogelsang H (2007) Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol Motil 19:279–287. https://doi.org/10.1111/j.1365-2982.2007.00900.x
Xiang Q, Tang X, Cui S, Zhang Q, Liu X, Zhao J, Zhang H, Mao B, Chen W (2022) Capsaicin, the spicy ingredient of chili peppers: effects on gastrointestinal tract and composition of gut microbiota at various dosages. Foods 11:686. https://doi.org/10.3390/foods11050686
Zhu Y, Peng W, Zhang J, Wang M, Firempong CK, Feng C, Liu H, Xu X, Yu J (2014) Enhanced oral bioavailability of capsaicin in mixed polymeric micelles: preparation, in vitro and in vivo evaluation. J Funct Foods 8:358–366. https://doi.org/10.1016/j.jff.2014.04.001
Lu M, Cao Y, Ho CT, Huang Q (2016) Development of organogel-derived capsaicin nanoemulsion with improved bioaccessibility and reduced gastric mucosa irritation. J Agric Food Chem 64:4735–4741. https://doi.org/10.1021/acs.jafc.6b01095
Perez-Burgos A, Wang L, Neufeld KAM, Mao YK, Ahmadzai M, Janssen LJ, Stanisz AM, Bienenstock J, Kunze WA (2015) The TRPV1 channel in rodents is a major target for antinociceptive effect of the probiotic Lactobacillus reuteri DSM 17938. J Physiol 593:3943–3957. https://doi.org/10.1113/jp270229
Pokusaeva K, Johnson C, Luk B, Uribe G, Fu Y, Oezguen N, Matsunami RK, Lugo M, Major A, Mori-Akiyama Y, Hollister EB, Dann SM, Shi XZ, Engler DA, Savidge T, Versalovic J (2017) GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neurogastroenterol Motil 29:1–14. https://doi.org/10.1111/nmo.12904
Hellström PM, Roos S, Nilsson K, Stanisz A, Forsythe P, Bienenstock J, Kunze W (2019) Translational studies of Lactobacillus gasseri effects on TRPV1 and motility, and therapeutic value in women with chronic constipation. Gastroenterology 156:S221. https://doi.org/10.1016/S0016-5085(19)37350-0
Tang T, Song J, Wang H, Zhang Y, Xin J, Suo H (2021) Qingke beta-glucan synergizes with a beta-glucan-utilizing Lactobacillus strain to relieve capsaicin-induced gastrointestinal injury in mice. Int J Biol Macromol 174:289–299. https://doi.org/10.1016/j.ijbiomac.2021.01.164
Dieleman LA, Palmen M, Akol H, Bloemena E, Pena AS, Meuwissen SGM, Rees EP (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391. https://doi.org/10.1046/j.1365-2249.1998.00728.x
Kang C, Zhang Y, Zhu X, Liu K, Wang X, Chen M, Wang J, Chen H, Hui S, Huang L, Zhang Q, Zhu J, Wang B, Mi M (2016) Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J Clin Endocrinol Metab 101:4681–4689. https://doi.org/10.1210/jc.2016-2786
Panpetch W, Visitchanakun P, Saisorn W, Sawatpanich A, Chatthanathon P, Somboonna N, Tumwasorn S, Leelahavanichkul A (2021) Lactobacillus rhamnosus attenuates Thai chili extracts induced gut inflammation and dysbiosis despite capsaicin bactericidal effect against the probiotics, a possible toxicity of high dose capsaicin. Plos One 16. https://doi.org/10.1371/journal.pone.0261189
Feng Y, Zhu Y, Wan J, Yang X, Firempong CK, Yu JN, Xu XM (2018) Enhanced oral bioavailability, reduced irritation and increased hypolipidemic activity of self-assembled capsaicin prodrug nanoparticles. J Funct Foods 44:137–145. https://doi.org/10.1016/j.jff.2018.03.006
Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T (2010) Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology 49:1215–1228. https://doi.org/10.1093/rheumatology/keq031
Smirnova MG, Kiselev SL, Gnuchev NV, Birchall JP, Pearson JP (2002) Role of the pro-inflammatory cytokines tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6 and interleukin-8 in the pathogenesis of the otitis media with effusion. Eur Cytokine Netw 13:161–172
Han J, Zhang S, Liu X, Xiao C (2019) Fabrication of capsaicin emulsions: Improving the stability of system and relieving the irritation to the gastrointestinal tract of rats. J Sci Food Agric 100:129–138. https://doi.org/10.1002/jsfa.10002
Chen Y, Mu J, Zhu M, Mukherjee A, Zhang H (2020) Transient receptor potential channels and inflammatory bowel disease. Front Immunol 11:180. https://doi.org/10.3389/fimmu.2020.00180
Drewes AM, Olesen AE, Farmer AD, Szigethy E, Rebours V, Olesen SS (2020) Gastrointestinal pain. Nat Rev Dis Primers 6:1–16. https://doi.org/10.1038/s41572-019-0135-7
Oliveira AP, Souza LKM, Araújo TSL, Araújo S, Nogueira KM, Sousa FBM, Silva RO, Pacífico DM, Martins CS, Brito GAC, Souza MHLP, Medeiros JVR (2019) Lactobacillus reuteri DSM 17938 Protects against gastric damage induced by ethanol administration in mice: role of TRPV1/substance P axis. Nutrients 11:208. https://doi.org/10.3390/nu11010208
Yang R, Zhao X, Wu W, Shi J (2021) Potential of probiotics for use as functional foods in patients with non-infectious gastric ulcer. Trends Food Sci Tech 111:463–474. https://doi.org/10.1016/j.tifs.2021.02.070
Suo H, Zhao X, Qian Y, Sun P, Zhu K, Li J, Sun BZ (2016) Lactobacillus fermentum suo attenuates HCl/ethanol induced gastric injury in mice through its antioxidant effects. Nutrients 8:17. https://doi.org/10.3390/nu8030155
Geppetti P, Trevisani M (2004) Activation and sensitisation of the vanilloid receptor: role in gastrointestinal inflammation and function. Br J Pharmacol 141:1313–1320. https://doi.org/10.1038/sj.bjp.0705768
Akbar A, Yiangou Y, Facer P, Walters JRF, Anand P, Ghosh S (2008) Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 57:923–929. https://doi.org/10.1136/gut.2007.138982
Holzer P (2008) TRPV1: a new target for treatment of visceral pain in IBS? Gut 57:882–884. https://doi.org/10.1136/gut.2008.149724
De Giorgio R, Tazzari PL, Barbara G, Stanghellini V, Corinaldesi R (1998) Detection of substance P immunoreactivity in human peripheral leukocytes. J Neuroimmunol 82:175–181. https://doi.org/10.1016/s0165-5728(97)00201-4
Esquerre N, Basso L, Defaye M, Vicentini FA, Cluny N, Bihan D, Hirota SA, Schick A, Jijon HB, Lewis IA, Geuking MB, Sharkey KA, Altier C, Nasser Y (2020) Colitis-Induced microbial perturbation promotes postinflammatory visceral hypersensitivity. Cell Mol Gastroenterol Hepatol 10:225–244. https://doi.org/10.1016/j.jcmgh.2020.04.003
Farup PG, Rudi K, Hestad K (2016) Faecal short-chain fatty acids - a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol 16:51. https://doi.org/10.1186/s12876-016-0446-z
Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S (2010) Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil 22:512–521. https://doi.org/10.1111/j.1365-2982.2009.01427.x
Ringel-Kulka T, Choi CH, Temas D, Kim A, Maier DM, Scott K, Galanko JA, Ringel Y (2015) Altered colonic bacterial fermentation as a potential pathophysiological factor in irritable bowel syndrome. Am J Gastroenterol 110:1339–1346. https://doi.org/10.1038/ajg.2015.220
Reeves AE, Theriot CM, Bergin IL, Huffnagle GB, Schloss PD, Young VB (2011) The interplay between microbiome dynamics and pathogen dynamics in a murine model of Clostridium difficile infection. Gut Microbes 2:145–158. https://doi.org/10.4161/gmic.2.3.16333
Fujio-Vejar S, Vasquez Y, Morales P, Magne F, Vera-Wolf P, Ugalde JA, Navarrete P, Gotteland MT (2017) he gut microbiota of healthy chilean subjects reveals a high abundance of the phylum Verrucomicrobia. Front Microbiol 8:1221. https://doi.org/10.3389/fmicb.2017.01221
Chen Y, Jin Y, Stanton C, Ross RP, Wang Z, Zhao J, Zhang H, Yang B, Chen W (2020) Dose-response efficacy and mechanisms of orally administered CLA-producing Bifidobacterium breve CCFM683 on DSS-induced colitis in mice. J Funct Foods 75:104245. https://doi.org/10.1016/j.jff.2020.104245
Chen Y, Wu H, Wu S, Lu N, Wang YT, Liu HN, Dong L, Liu TT, Shen XZ (2018) Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J Gastroenterol Hepatol 33:1844–1852. https://doi.org/10.1111/jgh.14281
Setoyama H, Imaoka A, Ishikawa H, Umesaki Y (2003) Prevention of gut inflammation by Bifidobacterium in dextran sulfate-treated gnotobiotic mice associated with Bacteroides strains isolated from ulcerative colitis patients. Microb Infect 5:115–122. https://doi.org/10.1016/s1286-4579(02)00080-1
Zheng J, Hoffman KL, Chen JS, Shivappa N, Sood A, Browman GJ, Dirba DD, Hanash S, Wei P, Hebert JR, Petrosino JF, Schembre SM, Daniel CR (2020) Dietary inflammatory potential in relation to the gut microbiome: results from a cross-sectional study. Br J Nutr 124:931–942. https://doi.org/10.1017/s0007114520001853
Wang Y, Li Q, Zha X, Luo JP (2022) Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct 13:143–160. https://doi.org/10.1039/d1fo03003e
Fang D, Shi D, Lv L, Gu S, Wu W, Chen Y, Guo J, Li A, Hu X, Guo F, Ye J, Li Y, Li L (2017) Bifidobacterium pseudocatenulatum LI09 and Bifidobacterium catenulatum LI10 attenuate D-galactosamine-induced liver injury by modifying the gut microbiota. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-09395-8
Sun Y, Wu D, Zeng W, Chen Y, Guo M, Lu B, Li H, Sun C, Yang L, Jiang X, Gao Q (2021) The role of intestinal dysbacteriosis induced arachidonic acid metabolism disorder in inflammaging in atherosclerosis. Front Cell Infect Microbiol 11:618265. https://doi.org/10.3389/fcimb.2021.618265
Tian B, Zhang Z, Zhao J, Ma QY, Liu HC, Nie CX, Ma ZY, An W, Li JX (2021) Dietary whole Goji berry (Lycium barbarum) intake improves colonic barrier function by altering gut microbiota composition in mice. Int J Food Sci Technol 56:103–114. https://doi.org/10.1111/ijfs.14606
Cao C, Tang M, Zhao N, Dong SY, Wu HH (2021) Effects of fish protein with glycation extent on gut microbiota and colonic barrier function in mice fed a high-fat diet. J Funct Foods 85:104636. https://doi.org/10.1016/j.jff.2021.104636
Min G, Zhuoyu L (2019) Polysaccharides isolated from nostoc commune vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct 10:6873–6881. https://doi.org/10.1039/c9fo00296k
Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A (2015) A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64:872–883. https://doi.org/10.1136/gutjnl-2014-307142
Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS (2019) Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) 47:365–371. https://doi.org/10.1016/j.aller.2018.12.009
Wang S, Chen H, Wen X, Mu J, Sun M, Song X, Liu B, Chen J, Fan X (2021) The efficacy of fecal microbiota transplantation in experimental autoimmune encephalomyelitis: transcriptome and gut microbiota profiling. J Immunol Res 2021:4400428. https://doi.org/10.1155/2021/4400428
Chai M, Wang L, Li X, Zhao J, Zhang H, Wang G, Chen W (2021) Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Funct 12:6058–6069. https://doi.org/10.1039/D1FO00559F
Yi R, Peng P, Zhang J, Muying D, Lingxia L, Yu Q, Jie Z (2019) Xin Z 2019 Lactobacillus plantarum CQPC02-fermented soybean milk improves loperamide-induced constipation in mice. J Med Food 22:1208–1221
Acknowledgements
The authors wish to thank the staff at the Animal Ethics Committee of Jiangnan University for their technical assistance during this study.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31972086, 32172173, and 32072197), the Yongjiang Talent Introduction Programme (2021C-003-T), and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author information
Authors and Affiliations
Contributions
Conceptualization: Bingyong Mao, Shumao Cui, and Hao Zhang. Investigation: Bingyong Mao, Qunran Xiang, Xin Tang, and Qiuxiang Zhang. Data analysis: Qunran Xiang, Xin Tang, and Xiaoming Liu. Writing original draft: Bingyong Mao and Qunran Xiang. Writing—reviewing and editing: Bingyong Mao, Jiangxin Zhao, and Shumao Cui. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, B., Xiang, Q., Tang, X. et al. Lactobacillus reuteri CCFM1175 and Lactobacillus paracasei CCFM1176 Could Prevent Capsaicin-Induced Ileal and Colonic Injuries. Probiotics & Antimicro. Prot. 15, 797–812 (2023). https://doi.org/10.1007/s12602-023-10106-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-023-10106-1