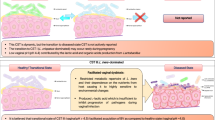
Abstract
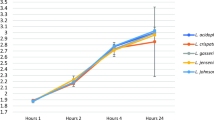
Lactobacilli, the most common group of bacteria found in a healthy vaginal microbiota, have been demonstrated to act as a defence against colonisation and overgrowth of vaginal pathogens. These groups of bacteria have sparked interests in incorporating them as probiotics aimed at re-establishing balance within the urogenital ecosystem. In this study, the safety characteristics of Limosilactobacillus reuteri 29B (L29B) strain were evaluated through whole genome sequencing (WGS) and animal study. Cell culture assay and 16S rDNA analysis were done to evaluate the ability of the strain to colonise and adhere to the mouse vaginal tract, and RAST analysis was performed to screen for potential genes associated with probiotic trait. The histological study on the mice organs and blood analysis of the mice showed there was no incidence of inflammation. We also found no evidence of bacterial translocation. The cell culture assay on HeLa cells showed 85% of adhesion, and there was a significant reduction of Candida strain viability in displacement assay. As for the 16S rDNA analysis, there was a significant amount of L29B colonisation of the vaginal microflora. Taken together, the intravaginal administration of L29B significantly reduced the number Enterobacteriaceae and Staphylococcaceae that were present in mouse vaginal tract. It also improved and promoted a balanced vaginal microflora environment without causing any harm or irritation to mice. Limosilactobacillus 29B (L29B) is safe to be administered intravaginally.












Similar content being viewed by others
References
Hill C et al (2014) Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Ravel J et al (2011) Vaginal microbiome of reproductive-age women. PNAS 108(1):4680–4687. https://doi.org/10.1073/pnas.1002611107
Srinivasan S et al (2010) Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One 5(4):e10197. https://doi.org/10.1371/journal.pone.0010197
Reid G (2008) Probiotic Lactobacilli for urogenital health in women. J Clin Gastroenterol 42 (3):234–236. https://doi.org/10.1097/mcg.0b013e31817f1298
Martín R, Soberón N, Vázquez F, Suárez JE (2008) La microbiota vaginal: composición, papel protector, patología asociada y perspectivas terapéuticas. Enferm Infecc Microbiol Clin 26(3):160–167. https://doi.org/10.1157/13116753
De Gregorio PR, Juárez Tomás MS, Leccese Terraf MC, Nader-Macías MEF (2015) Preventive effect of Lactobacillus reuteri CRL1324 on Group B Streptococcus vaginal colonization in an experimental mouse model. J Appl Microbiol 118(4):1034–1047.https://doi.org/10.1111/jam.12739
Ocaña VS, Elena B, De Holgado AAPR, Nader-Macias ME (1999) Surface characteristics of lactobacilli isolated from human vagina. J Gen Appl Microbiol 45(5):203–212. https://doi.org/10.2323/jgam.45.203
Tomás MSJ, Ocaña VS, Wiese B, Nader-Macías ME (2003) Growth and lactic acid production by vaginal Lactobacillus acidophilus CRL 1259, and inhibition of uropathogenic Escherichia coli. J Med Microbiol 52(12):1117–1124. https://doi.org/10.1099/jmm.0.05155-0
Borges S, Silva J, Teixeira P (2014) The role of lactobacilli and probiotics in maintaining vaginal health. Arch Gynecol Obstet 289(3):479–489. https://doi.org/10.1007/s00404-013-3064-9
Salminen S et al (1998) Demonstration of safety of probiotics - a review. Int J Food Microbiol 44(1–2):93–106. https://doi.org/10.1016/S0168-1605(98)00128-7
Kukkonen K et al (2008) Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics 122(1):8–12. https://doi.org/10.1542/PEDS.2007-1192
Srinivasan R, Meyer R, Padmanabhan R, Britto J (2006) Clinical safety of Lactobacillus casei shirota as a probiotic in critically ill children. J Pediatr Gastroenterol Nutr 42(2):171–173. https://doi.org/10.1097/01.MPG.0000189335.62397.CF
Ladas EJ et al (2015)The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant. 2016 512 51(2):262–266. https://doi.org/10.1038/bmt.2015.275
Ishibashi N, Yamazaki S (2001) Probiotics and safety. Am J Clin Nutr 73(2 SUPPL.):1–6. https://doi.org/10.1093/ajcn/73.2.465s
Salminen MK et al (2002) Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 35(10):1155–1160. https://doi.org/10.1086/342912/2/35-10-1155-FIG002.GIF
Meini S et al (2015) Breakthrough Lactobacillus rhamnosus GG bacteremia associated with probiotic use in an adult patient with severe active ulcerative colitis: case report and review of the literature. Infection 43(6):777–781. https://doi.org/10.1007/S15010-015-0798-2/TABLES/2
Kochan P et al (2011) Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—is the time right to revise probiotic safety guidelines.https://doi.org/10.1111/j.1469-0691.2011.03614.x
De Groote MA, Frank DN, Dowell E, Glode MP, Pace NR (2005) Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Dis J 24(3):278–280. https://doi.org/10.1097/01.INF.0000154588.79356.E6
Reid G, Gadir AA, Dhir R (2019) Probiotics: reiterating what they are and what they are not. Front Microbiol vol 10. no. MAR, p424. https://doi.org/10.3389/FMICB.2019.00424/BIBTEX
Sulin C, How CB, Jamil AAM, Yih CS, Meleh HU, Lung LTT (2019) Characterisation of the probiotic qualities exhibited by lactobacilli strains isolated from the anogenital tract. Malaysian J Med Heal Sci 15(7):37–45
Łukasik J, Salminen S, Szajewska H (2018) Rapid review shows that probiotics and fermented infant formulas do not cause d-lactic acidosis in healthy children. Acta Paediatr Int J Paediatr 107(8):1322–1326. https://doi.org/10.1111/APA.14338
Meleh HU et al (2020) Isolation and safety characterisation of lactobacilli strains with antimicrobial properties as potential probiotics for human use. Lwt, vol. 131, no. November 2019, p. 109796. https://doi.org/10.1016/j.lwt.2020.109796
Pohanka M (2020) D-lactic acid as a metabolite: toxicology, diagnosis, and detection. Biomed Res Int 2020:9. https://doi.org/10.1155/2020/3419034
Coman MM et al (2015) In vitro evaluation on HeLa cells of protective mechanisms of probiotic lactobacilli against Candida clinical isolates. J Appl Microbiol 119(5):1383–1390. https://doi.org/10.1111/jam.12947
Conti HR, Huppler AR, Whibley N, Gaffen SL (2014) Animal models for candidiasis, Curr. Protoc. Immunol, vol. 105, no. 19.6.1–19.6.17, pp. 1–23. https://doi.org/10.1002/0471142735.im1906s105
Dhanani AS, Bagchi T (2013) The expression of adhesin EF-Tu in response to mucin and its role in Lactobacillus adhesion and competitive inhibition of enteropathogens to mucin. J Appl Microbiol 115(2):546–554. https://doi.org/10.1111/JAM.12249
Granato D, Bergonzelli GE, Pridmore RD, Marvin L, Rouvet M, Corthésy-Theulaz IE (2004) Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect Immun 72(4):2160–2169. https://doi.org/10.1128/IAI.72.4.2160-2169.2004
Westra ER, Buckling A, Fineran PC (2014) CRISPR–Cas systems: beyond adaptive immunity,” Nat. Rev. Microbiol. 2014 125, vol. 12, no. 5, pp. 317–326, Apr. https://doi.org/10.1038/nrmicro3241
Whelan FJ, Surette MG (2017) A comprehensive evaluation of the sl1p pipeline for 16S rRNA gene sequencing analysis. Microbiome 5(1):100. https://doi.org/10.1186/s40168-017-0314-2
Crow JF, Dove WF (2001) Perspectives anecdotal, historical and critical commentaries on genetics Shannon’s brief foray into genetics. Genetics 159:915–917, [Online]. Available: https://academic.oup.com/genetics/article/159/3/915/6049509
Havenaar R, Ten Brink B, Huis In ’t Veld JHJ (1992) Selection of strains for probiotic use. Springer
Liong MT (2008) Safety of probiotics: translocation and infection. Nutr Rev 66(4):192–202. https://doi.org/10.1111/j.1753-4887.2008.00024.x
Zielińska D, Sionek B, Kołożyn-Krajewska D (2018) “Safety of probiotics”, in In Handbook of Food Bioengineering. Microbiome and Health, Academic Press, Diet, pp 131–161
Salminen S, Ouwehand A, Benno Y, Lee YK (1999) Probiotics: how should they be defined? Trends Food Sci Technol 10(3):107–110. https://doi.org/10.1016/S0924-2244(99)00027-8
Piddock LJV (2006) Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol 4(8):629–636. https://doi.org/10.1038/NRMICRO1464
Muñoz-Atienza E et al (2013) Antimicrobial activity, antibiotic susceptibility and virulence factors of lactic acid bacteria of aquatic origin intended for use as probiotics in aquaculture. BMC Microbiol 13(1):15. https://doi.org/10.1186/1471-2180-13-15
Vidal K, Donnet-Hughes A, Granato D (2002) Lipoteichoic acids from Lactobacillus johnsonii strain La1 and Lactobacillus acidophilus strain La10 antagonize the responsiveness of human intestinal epithelial HT29 cells to lipopolysaccharide and gram-negative bacteria. Infect Immun 70(4):2057–2064. https://doi.org/10.1128/IAI.70.4.2057-2064.2002/ASSET/A85D47FC-9C9C-49F3-B33A-40567B6C98AC/ASSETS/GRAPHIC/II0421368005.JPEG
Campedelli I et al (2019) Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl Environ Microbiol 85(1):1–21. https://doi.org/10.1128/AEM.01738-18
Ventola CL (2015) The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther 40(4):277
Salvetti E, O’Toole PW (2017) When regulation challenges innovation: the case of the genus Lactobacillus. Trends Food Sci Technol 66:187–194. https://doi.org/10.1016/J.TIFS.2017.05.009
Sun D, Jeannot K, Xiao Y, Knapp CW (2019) Editorial: Horizontal gene transfer mediated bacterial antibiotic resistance, Front. Microbiol, vol. 10, no. AUG, p. 1933. https://doi.org/10.3389/FMICB.2019.01933/XML/NLM
Boucard AS, Florent I, Polack B, Langella P, Bermúdez-Humarán LG (2022) Genome sequence and assessment of safety and potential probiotic traits of Lactobacillus johnsonii CNCM I-4884, Microorganisms, vol. 10, no. 2. https://doi.org/10.3390/MICROORGANISMS10020273
Marraffini LA, Sontheimer EJ (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA, Science (80-. ), vol. 322, no. 5909, pp. 1843–1845. https://doi.org/10.1126/science.1165771
Chen S, Yao Y, Zhang Y, Fan G (2020) CRISPR system: discovery, development and off-target detection, Cell Signal vol. 70, no., p. 109577. https://doi.org/10.1016/j.cellsig.2020.109577
Tao S, Chen H, Li N, Wang T, Liang W (2022) The spread of antibiotic resistance genes in vivo model. Can J Infect Dis Med Microbiol vol. 2022. https://doi.org/10.1155/2022/3348695
Palmer KL, Gilmore MS (2010) Multidrug-resistant enterococci lack CRISPR-cas, MBio, vol. 1, no. 4, Oct. https://doi.org/10.1128/MBIO.00227-10/SUPPL_FILE/MBIO00227-10-SF03.PDF
Steffen EK, Berg RD (1983) Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun 39(3):1252–1259. https://doi.org/10.1128/IAI.39.3.1252-1259.1983
Daniele M, Pascual L, Barberis L (2014) Curative effect of the probiotic strain Lactobacillus fermentum L23 in a murine model of vaginal infection by gardnerella vaginalis. Lett Appl Microbiol 59(1):93–98. https://doi.org/10.1111/lam.12249
Jang SE, Jeong JJ, Choi SY, Kim H, Han MJ, Kim DH (2017) Lactobacillus rhamnosus HN001 and lactobacillus acidophilus La-14 attenuate Gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients 9(6):1–14. https://doi.org/10.3390/nu9060531
De Gregorio PR, Juárez Tomás MS, Santos V, Nader-Macias MEF (2012) Beneficial lactobacilli: effects on the vaginal tract in a murine experimental model, Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol, vol. 102, no. 4, pp. 569–580. https://doi.org/10.1007/s10482-012-9752-9
FDA (2019) Generally Recognized as Safe (GRAS) | FDA. https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras. Accessed 14 Oct 2021
Meysick KC, Garber GE (1992) Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J Parasitol 78(1):157–160. https://doi.org/10.2307/3283708
Redondo-Lopez V, Cook RL, Sobel JD (1990) Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis 12(5):856–872. https://doi.org/10.1093/CLINIDS/12.5.856
Oakley BB, Fiedler TL, Marrazzo JM, Fredricks DN (2008) Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl Environ Microbiol 74(15):4898–4909. https://doi.org/10.1128/AEM.02884-07
Easmon CSF (2001) Understanding the bacterial flora of the female genital tract. J Obstet Gynaecol (Lahore) 32(4):69–77. https://doi.org/10.1086/318710
Davis CP (1996) Medical microbiology, in Normal Flora, University of Texas Medical Branch at Galveston, p. Chapter 6
Boris S, Suárez JE, Vázquez F, Barbés C (1998) Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 66(5):1985–1989. https://doi.org/10.1128/IAI.66.5.1985-1989.1998/ASSET/6D9A5EE5-2849-49BD-BACF-0EA84CCD287B/ASSETS/GRAPHIC/II0581383004.JPEG
Saunders S, Bocking A, Challis J, Reid G (2007) Effect of Lactobacillus challenge on Gardnerella vaginalis biofilms. Colloids Surfaces B Biointerfaces 55(2):138–142. https://doi.org/10.1016/J.COLSURFB.2006.11.040
Strus M, Kucharska A, Kukla G, Brzychczy-Włoch M, Maresz K, Heczko PB (2005) The in vitro activity of vaginal Lactobacillus with probiotic properties against Candida. Infect Dis Obstet Gynecol 13(2):69–75. https://doi.org/10.1080/10647440400028136
Vitali B, Cruciani F, Picone G, Parolin C, Donders G, Laghi L (2015) Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur J Clin Microbiol Infect Dis 34(12):2367–2376. https://doi.org/10.1007/s10096-015-2490-y
Mastromarino P et al (2002) Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J Appl Microbiol 93(5):884–893. https://doi.org/10.1046/J.1365-2672.2002.01759.X
Aroutcheva AA, Simoes JA, Faro S (2001) Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits gardnerella vaginalis. Infect Dis Obstet Gynecol 9(1):33–39. https://doi.org/10.1155/S1064744901000060
FAO (2006) Probiotics in food Health and nutritional properties and guidelines for evaluation FAO FOOD AND NUTRITION PAPER, vol. 85
Spencer RJ, Chesson A (1994) The effect of Lactobacillus spp. on the attachment of enterotoxigenic Escherichia coli to isolated porcine enterocytes. J Appl Bacteriol 77(2):215–220. https://doi.org/10.1111/J.1365-2672.1994.TB03066.X
Reid G (2000) In vitro testing of Lactobacillus acidophilus NCFM(TM) as a possible probiotic for the urogenital tract. Int Dairy J 10(5–6):415–419. https://doi.org/10.1016/S0958-6946(00)00059-5
Vintiñi E, Ocaña V, Elena Nader-Macías M (2004) Effect of Lactobacilli administration in the vaginal tract of mice: evaluation of side effects and local immune response by local administration of selected strains, in Public Health Microbiology, vol. 268, no. 1, New Jersey: Humana Press, pp. 401–410
Valverde JR, Mellado RP (2013) Analysis of metagenomic data containing high biodiversity levels, PLoS One, vol. 8, no. 3, p. e58118. https://doi.org/10.1371/JOURNAL.PONE.0058118
Beaumont M et al (2016) Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol 17(1):1–19. https://doi.org/10.1186/S13059-016-1052-7/FIGURES/5
Zárate G, Santos V, Nader-Macias ME (2007) Protective effect of vaginal Lactobacillus paracasei CRL 1289 against urogenital infection produced by Staphylococcus aureus in a mouse animal model. Infect Dis Obstet Gynecol 2007:48358. https://doi.org/10.1155/2007/48358
McGrory T, Garber GE (1992) Mouse intravaginal infection with Trichomonas vaginalis and role of Lactobacillus acidophilus in sustaining infection. Infect Immun vol. 60, no. 6, p. 2375. https://doi.org/10.1128/iai.60.6.2375-2379.1992
Homayouni A et al (2014) Effects of probiotics on the recurrence of bacterial vaginosis: a review. J Low Genit Tract Dis 18(1):79–86. https://doi.org/10.1097/LGT.0B013E31829156EC
Cribby S, Taylor M, Reid G (2008) Vaginal microbiota and the use of probiotics. Interdiscip Perspect Infect Dis 2008:1–9. https://doi.org/10.1155/2008/256490
Pascual L, Ruiz F, Giordano W, Barberis IL (2010) Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. J Med Microbiol 59(3):360–364. https://doi.org/10.1099/jmm.0.012583-0
Shahzadi K, Ahmad SZ, Ahmad SS, Arshad N (2020) Lactobacillus reuteri can reduce Gardnerella induced bacterial vaginosis in mice and modulate immune markers. BMC Infect Dis pp. 1–23. https://doi.org/10.21203/rs.3.rs-23647/v1
Verdenelli MC et al (2016) Impact of probiotic SYNBIO® administered by vaginal suppositories in promoting vaginal health of apparently healthy women. Curr Microbiol 73(4):483–490. https://doi.org/10.1007/s00284-016-1085-x
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2008) Genes and molecules of Lactobacilli supporting probiotic action. Microbiol Mol Biol Rev 72(4):728–764. https://doi.org/10.1128/mmbr.00017-08
Lebeer S, Vanderleyden J, De Keersmaecker SCJ (2010) Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol 8(3):171–184. https://doi.org/10.1038/nrmicro2297
Funding
This research was funded by Universiti Putra Malaysia through the Geran Putra IPS (Grant number: GP-IPS/2018/9613300).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Premmala Rangasamy, Leslie Thian Lung Than, and Hooi Ling Foo. The first draft of the manuscript was written by Premmala Rangasamy, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of Universiti Putra Malaysia (UPM/IACUC/AUP-R030/2020).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rangasamy, P., Foo, H.L., Yusof, B.N.M. et al. Probiotic Strain Limosilactobacillus reuteri 29B is Proven Safe and Exhibits Potential Probiotic Traits in a Murine Vaginal Model. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10094-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10094-2