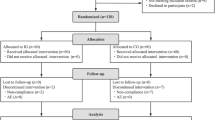
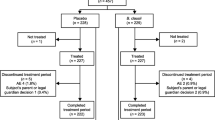
Abstract
Diarrhea is one of the most frequent side effects of antibiotic treatment and occurs in 25 to 40% of patients in use. One potential strategy to prevent this side effect is the concurrent use of probiotics. This study evaluated the efficacy of the strain Bifidobacterium lactis CCT 7858 in the prevention of diarrhea and improvement of gastrointestinal symptoms in hospitalized patients using antibiotics. This was a randomized, blinded, placebo-controlled clinical trial. This study included 104 patients in antibiotic treatment. Patients were randomized into two groups: placebo (maltodextrin) and intervention (strain Bifidobacterium lactis CCT 7858 at 9 × 1010 CFU concentration; GABBIA® Biotecnology, Santa Catarina, Brazil). Patients were supplemented depending on the duration of antibiotic therapy, and both were evaluated with scales in two moments: before and after treatment. We included 104 hospitalized patients. In follow-up, 38 (74.5%) of the B. lactis group have no reported diarrhea. In secondary outcomes, in five day strong abdominal distension was reported in 4 (7,3) placebo group and not reported in B. lactis. Abdominal noises, nausea, and vomiting were not registered in any group. B. lactis strain has been considered safe and with several benefits, including reduction of soft stools and gastrointestinal symptoms how abdominal noise, pain and distension, as well reduction of diarrhea.


Similar content being viewed by others
Availability of Data and Material
Data will be available on request.
References
McFarland LV (2008) Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol 3:563–578. https://doi.org/10.2217/17460913.3.5.563
Doron SI, Hibberd PL, Gorbach SL (2008) Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol 42:58–63. https://doi.org/10.1097/MCG.0b013e3181618ab7
Bartlett JG (2002) Clinical practice: antibiotic-associated diarrhea. N Engl J Med 346:334–339. https://doi.org/10.1056/NEJMcp011603
Spencer RC (1998) The role of antimicrobial agents in the aetiology of Clostridium difficile-associated disease. J Antimicrob Chemother Suppl C:21–27. https://doi.org/10.1093/jac/41.suppl_3.21
Bignardi GE (1998) Risk factors for Clostridium difficile infection. J Hosp Infect 40(1):1–15. https://doi.org/10.1016/s0195-6701(98)90019-6
Högenauer C, Hammer HF, Krejs GJ, Reisinger EC (1998) Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis 27:702–710. https://doi.org/10.1016/s0195-6701(98)90019-6
Aronsson B, Möllby R, Nord CE (1982) Clostridium difficile and antibiotic associated diarrhoea in Sweden. Scand J Infect Dis Suppl 35:53–58 PMID: 6962998
McFarland LV (2008) Updates on the changing epidemiology of Clostridium difficile- associated disease. Nat Clin Pract Gastroenterology Hepatol 5:40–48. https://doi.org/10.1038/ncpgasthep1029
Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA (2008) Antimicrobial associated risk factors for Clostridium difficile infection. Clin Infect Dis 2:19–31. https://doi.org/10.1086/521859
Hicks C, Moore S, Andrade A, Gentry C, Taormina C (2013) Probiotics for the prevention of antibiotic-associated diarrhea. Kans 6:148–151. https://doi.org/10.17161/kjm.v6i4.11460
FAO/WHO (2001) Evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria, Food and Agriculture Organization of the United Nations and World Health Organization, joint FAO/WHO expert consultation group: Cordoba, Argentina. Available in: https://www.fao.org/publications/card/en/c/7c102d95-2fd5-5b22-8faf-f0b2e68dfbb6/ Accessed in: 08 March 2021
Yoshioka H, Iseki K, Fujita K (1983) Development and differences of intestinal flora in the neonatal period in breast-fed and bottle-fed infants. Pediatrics 72:317–321 PMID: 6412205
Benno Y, Sawada K, Mitsuoka T (1984) The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol 28:975–986. https://doi.org/10.1111/j.1348-0421.1984.tb00754.x
Pochart P, Marteau P, Bouhnik Y et al (1992) Survival of Bifidobacteria ingested via fermented milk during their passage through the human small intestine: an in vivo study using intestinal perfusion. Am J Clin Nutr 55:78–80. https://doi.org/10.1093/ajcn/55.1.78
Link-Amster H, Rochat F, Saudan KY et al (1994) Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol 10:55–64. https://doi.org/10.1111/j.1574-695X.1994.tb00011.x
Fukushima Y, Li ST, Hara H et al (1997) Effect of follow-up formula containing Bifidobacteria (NAN BF) on fecal flora and fecal metabolites in healthy children. Bioscience Microflora 16: 65–72. https://doi.org/10.12938/bifidus1996.16.65
Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32:920–924. https://doi.org/10.3109/00365529709011203
Zhou SY, Liu T, Qin HZ et al (2013) Characteristics of commonly used scales for diagnosis and treatment outcome evaluation in constipation. World Chin J Digestol 21:2611–2616. https://doi.org/10.11569/wcjd.v21.i25.2611
Svedlund J, Sjödin I, Dotevall G (1988) GSRS - A clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 33:129–134. https://doi.org/10.1007/BF01535722
Dimenäs E, Glise H, Hallerbäck B, Hernqvist H, Svedlund J, Wiklund I (1993) Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand J Gastroenterol 28:681–687. https://doi.org/10.3109/00365529309098272
Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FEM, Hughes AO (1992) Defecation frequency and timing, and stool form in the general population: a prospective study. Gut 33:818–824. https://doi.org/10.1136/gut.33.6.818
Drossman DA (2006) The functional gastrointestinal disorders and the Rome III. In: Drossman DA, Corazziari E, Spiller RC, Thompson WG, Delvaux M, Talley NJ, Whitehead WE (eds) Rome III: the functional gastrointestinal disorders, 3rd edn. Inc, McLean VA Degnon Associates, pp 1–29
Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, Johnston BC (2017) Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 12:6095. https://doi.org/10.1002/14651858.CD006095.pub4
Hempel S, Newberry SJ, Maher AR et al (2012) Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA 307:1959–1969. https://doi.org/10.1001/jama.2012.3507
Videlock EJ, Cremonini F (2012) Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther 35:1355–1369. https://doi.org/10.1111/j.1365-2036.2012.05104.x
Ritchie ML, Romanuk TN (2012) A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS One 7:34938. https://doi.org/10.1371/journal.pone.0034938
McFarland LV (2010) Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World J Gastroenterol 16:2202–2222. https://doi.org/10.3748/wjg.v16.i18.2202.
Johnston BC, Supina AL, Ospina M, Vohra S (2007) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2:CD004827. https://doi.org/10.1002/14651858.CD004827.pub2
Lin JS, Chiu YH, Lin NT et al (2009) Different effects of probiotic species/strains on infections in preschool children: a double-blind, randomized. https://doi.org/10.1002/14651858.CD004827.pub2
Binns CW, Lee AH, Harding H et al (2007) The CUPDAY study: prebiotic-probiotic milk product in 1–3-year-old children attending childcare centres. Acta Paediatr 96:1646–1650. https://doi.org/10.1111/j.1651-2227.2007.00508.x
Weizman Z, Asli G, Alsheikh A (2005) Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 115:5–9. https://doi.org/10.1542/peds.2004-1815
Saavedra JM, Abi-Hanna A, Moore N et al (2004) Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 79: 261–267. https://doi.org/10.1093/ajcn/79.2.261
Hojsak I, Snovak N, Abdovic S et al (2010) Lactobacillus GG in the prevention of gastrointestinal and respiratory tract infections in children who attend day care centers: a randomized, double-blind, placebo-controlled trial. Clin Nutr 29:312–316. https://doi.org/10.1016/j.clnu.2009.09.008
Chouraqui JP, Van Egroo LD, Fichot MC (2004) Acidified milk formula supplemented with Bifidobacterium lactis: impact on infant diarrhea in residential care settings. J Pediatr Gastroenterol Nutr 38:288–292. https://doi.org/10.1097/00005176-200403000-00011
Thibault H, Aubert-Jacquin C, Goulet O (2004) Effects of long-term consumption of a fermented infant formula (with Bifidobacterium breve c50 and Streptococcus thermophilus 065) on acute diarrhea in healthy infants. J Pediatr Gastroenterol Nutr 39:147–152. https://doi.org/10.1097/00005176-200408000-00004
Hatakka K, Savilahti E, Ponka A et al (2001) Effect of long-term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ 322:1327. https://doi.org/10.1136/bmj.322.7298.1327
Collinson S, Deans A, Padua-Zamora A, Gregorio GV, Li C, Dans LF, Allen SJ (2020) Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 12(12):CD003048. https://doi.org/10.1002/14651858
Kaku N, Matsumoto N, Sasaki D, Tsuda K, Kosai K, Uno N, Morinaga Y, Tagami A, Adachi S, Hasegawa H, Osaki M, Yanagihara K (2020) Effect of probiotics on gut microbiome in patients with administration of surgical antibiotic prophylaxis: a randomized controlled study. J Infect Chemother 26(8):795–801. https://doi.org/10.1016/j.jiac.2020.03.008
Ouwehand AC, Kirjavainen PV, Gronlund MM, Isolauri E, Salminen SJ (1999) Adhesion of probiotic microorganisms to intestinal mucus. Int Dairy J 9:623– 630. https://doi.org/10.1016/j.jiac.2020.03.008
Jungersen M, Wind A, Johansen E, Christensen JE, Stuer-Lauridsen B, Eskesen D (2014) The science behind the probiotic strain Bifidobacterium animalis subsp. lactis BB-12(®). Microorganisms 2:92–110. https://doi.org/10.3390/microorganisms2020092
Saavedra JM, Bauman NA, Oung I et al (1994) Feeding of Bifidobacterium bifidum and Streptococcus thermophilus to infants in hospital for prevention of diarrhoea and shedding of rotavirus. Lancet 344:1046–9. https://doi.org/10.1016/s0140-6736(94)91708-6
Hotta M, Sato Y, Iwata S et al (1987) Clinical effects of Bifidobacterium preparations on pediatric intractable diarrhea. Keio J Med 298–314. https://doi.org/10.1016/s0140-6736(94)91708-6
Saavedra JM (1995) Microbes to fight microbes: a not so novel approach to controlling diarrheal disease. J Pediatr Gastroenterol Nutr 21:125–129. https://doi.org/10.1016/s0140-6736(94)91708-6
Fukushima Y, Kawata Y, Hara H et al (1998) Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol 42:39–44. https://doi.org/10.1016/s0168-1605(98)00056-7
Vanderhoof JA, Young RJ (1998) Use of probiotics in childhood gastrointestinal disorders. J Pediatr Gastroenterol Nutr 27:323–332. https://doi.org/10.1097/00005176-199809000-00011
Oelschlaeger TA (2010) Mechanisms of probiotic actions – a review. Int J Med Microbiol 300:57–62. https://doi.org/10.1016/j.ijmm.2009.08.005
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2008) Mechanisms of action of probiotics: Recent advances. Inflamm Bowel Dis 1:300–310. https://doi.org/10.1002/ibd.20602
Waller PA, Gopal PK, Leyer GJ, Ouwehand AC, Reifer C, Stewart ME, Miller LE (2011) Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand J Gastroenterol 46:1057–1064. https://doi.org/10.3109/00365521.2011.584895
Tabbers MM, Chmielewska A, Roseboom MG, Boudet C, Perrin C, Szajewska H, Benninga MA (2009) Effect of the consumption of a fermented dairy product containing Bifidobacterium lactis DN-173 010 on constipation in childhood: a multicentre randomised controlled trial (NTRTC: 1571). BMC Pediatr 9:22. https://doi.org/10.1186/1471-2431-9-22
Funding
This study was funded by the Gabbia Biotechnology.
Author information
Authors and Affiliations
Contributions
MM, GFAJ: conceptualization and design of the study; data curation; formal analysis and methodology; roles/writing-original draft. EC, LC, CSS: conceptualization, data curation, methodology. APLV, MPR, FR: conceptualization and design of the study; methodology. CM, KM: statistical analyses; writing-review and editing. APV, MR, FR: design of the study, funding acquisition. DCD and FDP: conceptualization and design of the study; project administration; supervision; writing-review and editing. All authors approved final version submitted.
Corresponding author
Ethics declarations
Ethics Approval
The Ethics Committees of Hospital São José in Criciúma approved the study (Number: 4.449.750/2020 and CAAE number: 40641120.9.0000.5364) and all patients or proxies gave written informed consent, without any refusal. The study was registered in clinical trial = NCT04742322.
Consent to Participate
All patients or proxies gave written informed consent, without any refusal.
Consent for Publication
All authors approved final version submitted.
Conflict of Interest/Competing Interests
Gabbia Biotechnology are developing B. lactis strain for the commercial purposes. Gabriel Jesus, Marina Rosseto, Ana Paula Voytena, and Fernanda Ramlov are members of Gabbia Biotechnology. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michels, M., Córneo, E., Cucker, L. et al. Bifidobacterium lactis CCT 7858 Improves Gastrointestinal Symptoms by Antibiotics Treatment: a Double-Blind, Randomized, Placebo-Controlled Trial. Probiotics & Antimicro. Prot. 15, 738–748 (2023). https://doi.org/10.1007/s12602-021-09900-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09900-6