Abstract
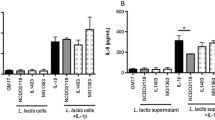
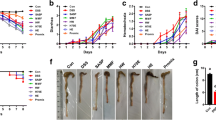
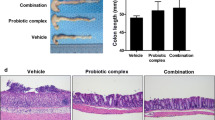
Currently, there are no effective therapeutic agents to limit intestinal mucosal damage associated with inflammatory bowel disease (IBD). Based on several clinical studies, probiotics have emerged as a possible novel therapeutic strategy for IBD; however, their possible mechanisms are still poorly understood. Although probiotics in murine and human improve disease severity, very little is known about the specific contribution of cell wall contents of probiotics in IBD. Herein, we investigated the protective effects of cell wall contents of three Lactobacillus species in lipopolysaccharide (LPS)-induced colitis rats. LPS-sensitized rats were rendered colitic by colonic instillation of LPS (500 µg/rat) for 14 consecutive days. Concurrently, cell wall contents isolated from 106 CFU of L. casei (LC), L. acidophilus (LA), and L. rhamnosus (LA) was given subcutaneously for 21 days, considering sulfasalazine (100 mg/kg, p.o.) as standard. The severity of colitis was assessed by body weight loss, food intake, stool consistency, rectal bleeding, colon weight/length, spleen weight, and histological analysis. Colonic inflammatory markers (myeloperoxidase activity, C-reactive protein, and pro-inflammatory cytokines) and oxidative stress markers (malondialdehyde, reduced glutathione, and nitric oxide) were also assayed. Cell wall contents of LC, LA, and LR significantly ameliorated the severity of colitis by reducing body weight loss and diarrhea and bleeding incidence, improving food intake, colon weight/length, spleen weight, and microscopic damage to the colonic mucosa. The treatment also reduced levels of inflammatory and oxidative stress markers and boosted anti-oxidant molecule. In conclusion, cell wall contents of LC, LA, and LR attenuate LPS-induced colitis by modulating immuno-inflammation and oxidative stress.





Similar content being viewed by others
References
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621. https://doi.org/10.1146/annurev-immunol-030409-101225
Toumi R, Soufli I, Rafa H, Belkhelfa M, Biad A, Touil-Boukoffa C (2014) Probiotic bacteria lactobacillus and bifidobacterium attenuate inflammation in dextran sulfate sodium-induced experimental colitis in mice. Int J Immunopathol Pharmacol 27(4):615–627. https://doi.org/10.1177/039463201402700418
Asquith MJ, Boulard O, Powrie F, Maloy KJ (2010) Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology 139(2):519–529. https://doi.org/10.1053/j.gastro.2010.04.045
Garrett WS, Gordon JI, Glimcher LH (2010) Homeostasis and inflammation in the intestine. Cell 140(6):859–870. https://doi.org/10.1016/j.cell.2010.01.023
Ahlawat S, Asha, Sharma KK (2020) Gut-organ axis: a microbial outreach and networking. Lett Appl Microbiol https://doi.org/10.1111/lam.13333
Geier MS, Butler RN, Howarth GS (2007) Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol 115(1):1–11. https://doi.org/10.1016/j.ijfoodmicro.2006.10.006
Mangerich A, Dedon PC, Fox JG, Tannenbaum SR, Wogan GN (2013) Chemistry meets biology in colitis-associated carcinogenesis. Free Radic Res 47(11):958–986. https://doi.org/10.3109/10715762.2013.832239
Stephens M, von der Weid PY (2019) Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 11(3):421–432. https://doi.org/10.1080/19490976.2019.1629235
Zhang L, Wei X, Zhang R, Si D, Petitte JN, Ahmad B, Zhang M (2019) A novel peptide ameliorates LPS-induced intestinal inflammation and mucosal barrier damage via its antioxidant and antiendotoxin effects. Int J Mol Sci 20(16):3974. https://doi.org/10.3390/ijms20163974
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr 10(suppl1):S49–S66. https://doi.org/10.1093/advances/nmy063
Grimoud J, Durand H, de Souza S et al (2010) In vitro screening of probiotics and synbiotics according to anti-inflammatory and anti-proliferative effects. Int J Food Microbiol 144(1):42–50. https://doi.org/10.1016/j.ijfoodmicro.2010.09.007
Vanderpool C, Yan F, Polk DB (2008) Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm Bowel Dis 14(11):1585–1596. https://doi.org/10.1002/ibd.20525
LeBlanc JG, del Carmen S, Miyoshi A et al (2011) Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J Biotechnol 151(3):287–293. https://doi.org/10.1016/j.jbiotec.2010.11.008
Sengul N, Isık S, Aslım B, Ucar G, Demirbag AE (2011) The effect of exopolysaccharide-producing probiotic strains on gut oxidative damage in experimental colitis. Dig Dis Sci 56(3):707–714. https://doi.org/10.1007/s10620-010-1362-7
Amaretti A, di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97(2):809–817. https://doi.org/10.1007/s00253-012-4241-7
Songisepp E, Kals J, Kullisaar T, Mandar R, Hutt P, Zilmer M, Mikelsaar M (2005) Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr J 4:22. https://doi.org/10.1186/1475-2891-4-22.10.1186/1475-2891-4-22
Ishikawa H, Akedo I, Umesaki Y, Tanaka R, Imaoka A, Otani T (2003) Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J Am Coll Nutr 22(1):56–63. https://doi.org/10.1080/07315724.2003.10719276
LoreaBaroja M, Kirjavainen PV, Hekmat S, Reid G (2007) Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol 149(3):470–479. https://doi.org/10.1111/j.1365-2249.2007.03434.x
Simren M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, Strid H (2010) Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome – a randomized, double-blind, controlled study. Aliment Pharmacol Ther 31(2):218–227. https://doi.org/10.1111/j.1365-2036.2009.04183.x
Haller D, Antoine JM, Bengmark S, Enck P, Rijkers GT, Lenoir-Wijnkoop I (2010) Guidance for substantiating the evidence for beneficial effects of probiotics: probiotics in chronic inflammatory bowel disease and the functional disorder irritable bowel syndrome. J Nutr 140(3):690S-697S. https://doi.org/10.3945/jn.109.113746
Steed H, Macfarlane GT, Blackett KL et al (2010) Clinical trial: the microbiological and immunological effects of synbiotic consumption - a randomized double-blind placebo-controlled study in active Crohn’s disease. Aliment Pharmacol Ther 32(7):872–883. https://doi.org/10.1111/j.1365-2036.2010.04417.x
Cha BK, Jung SM, Choi CH et al (2012) The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol 46(3):220–227. https://doi.org/10.1097/MCG.0b013e31823712b1
Niv E, Naftali T, Hallak R, Vaisman N (2005) The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome – a double blind, placebo-controlled, randomized study. Clin Nutr 24(6):925–931. https://doi.org/10.1016/j.clnu.2005.06.001
Bousvaros A, Guandalini S, Baldassano RN et al (2005) A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn’s disease. Inflamm Bowel Dis 11(9):833–839. https://doi.org/10.1097/01.mib.0000175905.00212.2c
Marteau P, Lemann M, Seksik P et al (2006) Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of postoperative recurrence in Crohn’s disease: a randomized, double blind, placebo controlled GETAID trial. Gut 55(6):842–847. https://doi.org/10.1136/gut.2005.076604
Chermesh I, Tamir A, Reshef R et al (2007) Failure of Synbiotic 2000 to prevent postoperative recurrence of Crohn’s disease. Dig Dis Sci 52(2):385–389. https://doi.org/10.1007/s10620-006-9549-7
Patel RB, Prajapati KD, Sonara BM et al (2014) Ameliorative potential of aliskiren in experimental colitis in mice. Eur J Pharmacol 737:70–76. https://doi.org/10.1016/j.ejphar.2014.05.009
Siegmund B, Rieder F, Albrich S et al (2001) Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther 296(1):99–105
Perez S, Talens-Visconti R, Rius-Perez S, Finamor I, Sastre J (2017) Redox signaling in the gastrointestinal tract. Free Radic Biol Med 104:75–103. https://doi.org/10.1016/j.freeradbiomed.2016.12.048
Podolsky DK (2002) Inflammatory bowel disease. N Engl J Med 347(6):417–429. https://doi.org/10.1056/NEJMra020831
Lebeer S, Claes IJJ, Vanderleyden J (2012) Anti-inflammatory potential of probiotics: lipoteichoic acid makes a difference. Trends Microbiol 20(1):5–10. https://doi.org/10.1016/j.tim.2011.09.004
Fessler MB, Malcolm KC, Duncan MW, Worthen GS (2002) A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J Biol Chem 277(35):31291–31302. https://doi.org/10.1074/jbc.M200755200
Torres-Rodríguez ML, García-Chavez E, Berhow M, de Mejia EG (2016) Anti-inflammatory and anti-oxidant effect of Calea urticifolia lyophilized aqueous extract on lipopolysaccharide-stimulated RAW 264.7 macrophages. J Ethnopharmacol 188:266–274. https://doi.org/10.1016/j.jep.2016.04.057
Liu M, Li S, Wang X, Zhu Y, Zhang J, Liu H, Jia L (2018) Characterization, anti-oxidation and anti-inflammation of polysaccharides by Hypsizygus marmoreus against LPS-induced toxicity on lung. Int J Biol Macromol 111:121–128. https://doi.org/10.1016/j.ijbiomac.2018.01.010
Hotta T, Yoshida N, Yoshikawa T, Sugino S, Kondo M (1986) Lipopolysaccharide-induced colitis in rabbits. Res Exp Med (Berl) 186(1):61–69. https://doi.org/10.1007/BF01851834
Im E, Riegler FM, Pothoulakis C, Rhee SH (2012) Elevated lipopolysaccharide in colon evokes intestinal inflammation, aggravated in immune modulator-impaired mice. Am J Physiol Gastrointest Liver Physiol 303(4):G490-497. https://doi.org/10.1152/ajpgi.00120.2012
Martin JC, Beriou G, Josien R (2016) Dextran sulfate sodium (DSS)-induced acute colitis in the rat. Methods Mol Biol 1371:197–203. https://doi.org/10.1007/978-1-4939-3139-2_12
Ashrafi F, Kowsari F, Darakhshandeh A, Adibi P (2014) Composite lymphoma in a patient with ulcerative colitis: a case report. Int J Hematol Oncol Stem Cell Res 8(4):45–48
Oustamanolakis P, Koutroubakis IE, Kouroumalis EA (2011) Diagnosing anemia in inflammatory bowel disease: beyond the established markers. J Crohns Colitis 5(5):381–391. https://doi.org/10.1016/j.crohns.2011.03.010
Krawisz JE, Sharon P, Stenson WF (1984) Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology 87(6):1344–1350
Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN (2014) Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS One 9(5):e97193. https://doi.org/10.1371/journal.pone.0097193
Zizzo MG, Caldara G, Bellanca A, Nuzzo D, Di Carlo M, Serio R (2018) Preventive effects of guanosine on intestinal inflammation in 2, 4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 27(2):349–359. https://doi.org/10.1007/s10787-018-0506-9
Cario E (2010) Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis 16(9):1583–1597. https://doi.org/10.1002/ibd.21282
Sproston NR, Ashworth JJ (2018) Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9:754. https://doi.org/10.3389/fimmu.2018.00754
Finamore A, Ambra R, Nobili F et al (2018) Redox role of Lactobacillus casei shirota against the cellular damage induced by 2,2′-azobis (2-amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front Immunol 9:1131. https://doi.org/10.3389/fimmu.2018.01131
Baghbani-Arani F, Asgary V, Hashemi A (2019) Cell-free extracts of Lactobacillus acidophilus and Lactobacillus delbrueckii display antiproliferative and antioxidant activities against HT-29 cell line. Nutr Cancer 11:1–10. https://doi.org/10.1080/01635581.2019.1685674
Lee SI, Kim HS, Koo JM, Kim IH (2016) Lactobacillus acidophilus modulates inflammatory activity by regulating the TLR4 and NF-κB expression in porcine peripheral blood mononuclear cells after lipopolysaccharide challenge. Br J Nutr 115(4):567–575. https://doi.org/10.1017/S0007114515004857
Funding
This work was supported by K. B. Institute of Pharmaceutical Education and Research, Gandhinagar, Gujarat, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chorawala, M.R., Chauhan, S., Patel, R. et al. Cell Wall Contents of Probiotics (Lactobacillus species) Protect Against Lipopolysaccharide (LPS)-Induced Murine Colitis by Limiting Immuno-inflammation and Oxidative Stress. Probiotics & Antimicro. Prot. 13, 1005–1017 (2021). https://doi.org/10.1007/s12602-020-09738-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09738-4