Abstract
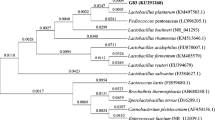
The past decade has brought a significant rise in antimicrobial resistance, and the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter species) have considerably aggravated a threat to public health, causing nosocomial infections worldwide. The objective of the current study was to isolate novel probiotic strain with antimicrobial activity against multidrug-resistant ESKAPE pathogens. For this purpose, eighteen breastfed infant faeces were collected and lactic acid bacteria (LAB) with antagonistic activity were isolated. Out of 102 anaerobic LAB isolated, only nine exhibited inhibitory activity against all ESKAPE pathogens. These selected nine isolates were further characterized for their probiotic attributes such as lysozyme tolerance, simulated gastrointestinal tolerance, cellular auto-aggregation and cell surface hydrophobicity. Bile salt deconjugation and cholesterol-lowering capacity was also determined. Among all nine, isolate LBM220 was found to possess superior probiotic potential. Confirmatory identification of isolate LBM220 was done by both 16S rRNA sequence analysis and mass spectrometric analysis using MALDI-TOF. Based on BLAST result, isolate LBM220 was identified as Lactobacillus gasseri. Phylogenetic analysis of Lactobacillus gasseri LBM220 [accession number MN097539] was performed. Also, detailed safety evaluation study of Lact. gasseri LBM220 showed the presence of intrinsic antibiotic resistance and the absence of hemolytic, DNase, gelatinase and toxic mucinolytic activity. Time kill assay was also performed to confirm the strong kill effect of Lact. gasseri LBM220 on all six multidrug resistant ESKAPE pathogens. Thus, Lact. gasseri LBM220 can be utilized and explored as potential probiotic with therapeutic intervention.




Similar content being viewed by others
References
Waclaw B (2016) Evolution of drug resistance in bacteria. Adv Exp Med Biol 915:49–67. https://doi.org/10.1007/978-3-319-32189-9_5
Stewardson AJ, Allignol A, Beyersmann J, Graves N, Schumacher M, Meyer R, Tacconelli E, de Angelis G, Farina C, Pezzoli F, Bertrand X, Gbaguidi-Haore H, Edgeworth J, Tosas O, Martinez JA, Ayala-Blanco MP, Pan A, Zoncada A, Marwick CA, Nathwani D, Seifert H, Hos N, Hagel S, Pletz M, Harbarth S, the TIMBER Study Group (2016) The health and economic burden of bloodstream infections caused by antimicrobial-susceptible and non-susceptible Enterobacteriaceae and Staphylococcus aureus in European hospitals, 2010 and 2011: a multicentre retrospective cohort study. Euro Surveill https://doi.org/10.2807/1560-7917.ES.2016.21.33.30319
Zhen X, Lundborg CS, Sun X, Hu X, Dong H (2019) Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob Resist Infect Control 8:137. https://doi.org/10.1186/s13756-019-0590-7
Pendleton JN, Gorman SP, Gilmore BF (2013) Clinical relevance of the ESKAPE pathogens. Expert Rev Anti-Infect Ther 11(3):297–308. https://doi.org/10.1586/eri.13.12
Santajit S, Indrawattana N (2016) Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int 2016:2475067–2475068. https://doi.org/10.1155/2016/2475067
FAO/WHO (2006) Probiotics in food, health and nutritional properties and guidelines for evaluation. Rome: FAO Food and Nutrition Paper. http://www.fao.org/3/a-a0512e.pdf
Wang L, Guo MJ, Gao Q, Yang JF, Yang L, Pang XL, Jiang XJ (2018) The effects of probiotics on total cholesterol: a meta-analysis of randomized controlled trials. Medicine. 10. 1097/MD.0000000000009679
Wang ZB, Xin SS, Ding LN, Ding WY, Hou YL, Liu CQ, Zhang XD (2019) The potential role of probiotics in controlling overweight/obesity and associated metabolic parameters in adults: a systematic review and meta-analysis. Evid Based Complement Alternat Med 2019:1–14. https://doi.org/10.1155/2019/3862971
Nazir Y, Hussain SA, Abdul HA, Song Y (2018) Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. Biomed Res Int. 10. 1155/2018/3428437
Rittiphairoj T, Pongpirul K, Mueller NT, Li T (2019) Probiotics for glycemic control in patients with type 2 diabetes mellitus: protocol for a systematic review. Syst Rev 8:227. https://doi.org/10.1186/s13643-019-1145-y
Domingo SJJ (2017) Review of the role of probiotics in gastrointestinal diseases in adults. Gastroenterol Hepato 40(6):417–429. https://doi.org/10.1016/j.gastre.2016.12.001
Kobatake E, Nakagawa H, Seki T, Miyazaki T (2017) Protective effects and functional mechanisms of Lactobacillus gasseri SBT2055 against oxidative stress. PLoS One. https://doi.org/10.1371/journal.,pone.0177106
Staudacher H (2015) Probiotics for lactose intolerance and irritable bowel syndrome. Br J Community Nurs Suppl Nutrition: S12–S14. https://doi.org/10.12968/bjcn.2015.20.Sup6a.S12
Thakur N, Rokana N, Panwar H (2016) Probiotics: selection criteria, safety and role in health and diseases. J Innov Biol 3:259–270 https://www.semanticscholar.org/paper/Probiotics-%3A-Selection-criteria-%2C-safety-and-role-Thakur-Rokana/10c2b5d9f103e4f87bfe01146916c053ec9e2835
Bejar W, Farhat-Khemakhen A, Smaoui S, Mohamed M, Ben M, Badis F, Lofti A, Emmanuelle M, Samir M, Hichem B, Hichem C (2011) Selection of Lactobacillus plantarum TN627 as a new probiotic candidate based on in vitro functional properties. Biotechnol Bioproc E 16:1115–1123. https://doi.org/10.1007/s12257-011-0198-0
Yu HJ, Chen YF, Yang HJ, Yang J, Li CK, Kwok LY, Zhang HP, Sun TG (2014) Screening for Lactobacillus plantarum with potential inhibitory activity against enteric pathogens. Ann Micro 65(3):1257–1265. https://doi.org/10.1007/s13213-014-0963-3
Sirichokchatchawan W, Pupa P, Praechansri P, Am-In N, Tanasupawat S, Sonthayanon P, Prapasarakul N (2018) Autochthonous lactic acid bacteria isolated from pig faeces in Thailand show probiotic properties and antibacterial activity against enteric pathogenic bacteria. Microb Pathog 119:208–215. https://doi.org/10.1016/j.micpath.2018.04.031
Turchi B, Mancini S, Fratini F, Pedonese F, Nuvoloni R, Bertelloni F, Ebani VV, Cerri D (2013) Preliminary evaluation of probiotic potential of Lactobacillus plantarum strains isolated from Italian food products. World J Microbiol Biotechnol 29(10):1912–1922. https://doi.org/10.1007/s11274-013-1356-7
de Moraes GMD, de Abreu LR, do Egito AS, Salles HO, da Silva LMF, Nero LA, Todorov SD, dos Santos KMO (2017) Functional properties of Lactobacillus mucosae strains isolated from brazilian goat milk. Probiotics & Antimicro Prot 9(3):235–245. https://doi.org/10.1007/s12602-016-9244-8
Shehata MG, Sohaimy SA, El-Malak A, El-Sahn YMM (2016) Screening of isolated potential probiotic lactic acid bacteria for cholesterol lowering property and bile salt hydrolase activity. Ann Agric Sci 61(1):65–75. https://doi.org/10.1016/j.aoas.2016.03.001
Rastogi S, Mittal V, Singh A (2019) In vitro evaluation of probiotic potential and safety assessment of Lactobacillus mucosae strains isolated from Donkey’s lactation. Probiotics and Antimicrob Proteins 12:1045–1056. https://doi.org/10.1007/s12602-019-09610-0
Zhang Y, Zhang L, Du M, Yi H, Guo C, Tuo Y, Han X, Li J, Zhang L, Yang L (2011) Antimicrobial activity against Shigella sonnei and probiotic properties of wild lactobacilli from fermented food. Microbiol Res 167(1):27–31. https://doi.org/10.1016/j.micres.2011.02.006
Clinical and Laboratory Standards Institute (2018) Performance standards for antimicrobial disk susceptibility tests; approved standard - 12th ed. M02-A13. Clinical and Laboratory Standards Institute, Wayne, PA https://clsi.org/standards/products/microbiology/documents/m02/
Wang C, Chang T, Yang H, Cui M (2015) Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 47:231–236. https://doi.org/10.1016/j.foodcont.2014.06.034
Abdelhamid AG, Esaam A, Hazaa MM (2018) Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli. Saudi Pharm J 26(5):603–607. https://doi.org/10.1016/j.jsps.2018.03.004
Tiengrim S, Thamlikitkul V (2012) Inhibitory activity of fermented milk with Lactobacillus casei strain Shirota against common multidrug-resistant bacteria causing hospital-acquired infections. J Med Assoc Thail 95(Suppl 2):S1–S5
Kumar M, Dhaka P, Vijay D, Vergis J, Mohan V, Kumar A, Kurkure NV, Barbuddhe SB, Malik SV, Rawool DB (2016) Antimicrobial effects of Lactobacillus plantarum and Lactobacillus acidophilus against multidrug-resistant enteroaggregative escherichia coli. Int J of Antimicrob Agents 48(3):265–270. https://doi.org/10.1016/j.ijantimicag.2016.05.014
Jampaphaeng K, Cocolin L, Maneerat S (2017) Selection and evaluation of functional characteristics of autochthonous lactic acid bacteria isolated from traditional fermented stinky bean (Sataw-Dong). Ann Microbiol 67(1):25–36. https://doi.org/10.1007/s13213-016-1233-3
Kudo H, Sasaki Y (2019) Intracellular pH determination for the study of acid tolerance of lactic acid bacteria: methods and protocols. Methods Mol Biol. https://doi.org/10.1007/978-1-4939-8907-2_4
Begley M, Hill C, Gahan CG (2006) Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72(3):1729–1738. https://doi.org/10.1128/AEM.72.3.1729-1738.2006
Rodrigues da Cunha L, Fortes Ferreira CL, Durmaz E, Goh YJ, Sanozky-Dawes R, Klaenhammer T (2012) Characterization of Lactobacillus gasseri isolates from a breast-fed infant. Gut Microbes 3:15–24. https://doi.org/10.4161/gmic.19489
Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Jahromi MF, Ho YW (2014) Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res Int 2014:1–16. https://doi.org/10.1155/2014/927268
Kumari A, Angmo K, Monika BTC (2016) Probiotic attributes of indigenous Lactobacillus spp. isolated from traditional fermented foods and beverages of north-western Himalayas using in vitro screening and principal component analysis. J Food Sci Technol 53(5):2463–2475. https://doi.org/10.1007/s13197-016-2231-y
Collado MC, Meriluoto J, Salminen S (2008) Adhesion and aggregation properties of probiotic and pathogen strains. Eur Food Res Technol 226:1065–1073. https://doi.org/10.1007/s00217-007-0632
Puniya M, Kumar RM, Panwar H, Kumar N, Ramneek P, AK (2016) Screening of lactic acid bacteria of different origin for their probiotic potential. J Food Process Technol https://doi.org/10.4172/2157-7110.1000545, 07
Boris S, Suárez JE, Vázquez F, Barbés C (1998) Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect Immun 66:1985–1989. https://doi.org/10.1128/IAI.66.5.1985-1989.1998
Kassaa IA, Hamze M, Hober D, Chihib NE, Drider D (2014) Identification of vaginal lactobacilli with potential probiotic properties isolated from women in north Lebanon. Microb Ecol 67:722–734
Šrka H, Milada P, Kateřina D (2017) Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol Adv 36(3):682–690. https://doi.org/10.1016/j.biotechadv.2017.12.005
Ishimwe N, Daliri EB, Lee BH, Fang F, Du G (2015) The perspective on cholesterol-lowering mechanisms of probiotics. Mol Nutr Food Res 59(1):94–105. https://doi.org/10.1002/mnfr.201400548
Costabile A, Buttarazzi I, Kolida S, Quercia S, Baldini J, Swann JR, Brigidi P, Gibson GR (2017) An in vivo assessment of the cholesterol-lowering efficacy of Lactobacillus plantarum ECGC 13110402 in normal to mildly hypercholesterolaemic adults. Plos One. https://doi.org/10.1371/journal.,pone.0187964
Sharma C, Gulati S, Thakur N, Singh BP, Gupta S, Kaur S, Mishra SK, Puniya AK, Gill JPS, Panwar H (2017) Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples. 3 Biotech. https://doi.org/10.1007/s13205-017-0682-0
Nawaz M, Wang J, Zhou A, Ma C, Wu X, Moore JE, Millar BC, Xu J (2011) Characterization and transfer of antibiotic resistance in lactic acid bacteria from fermented food products. Curr Microbiol 62(3):1081–1089. https://doi.org/10.1007/s00284-010-9856-2
Dec M, Urban-Chmiel R, Stępień-Pyśniak D, Wernicki A (2017) Assessment of antibiotic susceptibility in Lactobacillus isolates from chickens. Gut Pathog 9:54. https://doi.org/10.1186/s13099-017-0203-z
Jiang M, Zhang F, Wan C, Xiong Y, Shah NP, Wei H, Tao X (2016) Evaluation of probiotic properties of Lactobacillus plantarum WLPL04 isolated from human breast milk. J Dairy Sci 99(3):1736–1746. https://doi.org/10.3168/jds.2015-10434
Kõll P, Mändar R, Smidt I, Hütt P, Truusalu K, Mikelsaar RH, Shchepetova J, Krogh-Andersen K, Marcotte H, Hammarström L, Mikelsaar L (2010) Screening and evaluation of human intestinal lactobacilli for the development of novel gastrointestinal probiotics. Curr Microbiol 61(6):560–566. https://doi.org/10.1007/s00284-010-9653-y
Martín R, Jiménez E, Olivares M, Marín ML, Fernández L, Xaus J, Rodríguez JM (2006) Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother-child pair. Int J Food Microbiol 112(1):35–43. https://doi.org/10.1016/j.ijfoodmicro.2006.06.011
Gaucher F, Bonnassie S, Rabah H, Marchand P, Blanc P, Jeantet B, Jan G (2019) Review: adaptation of beneficial propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front Microbiol https://doi.org/10.3389/fmicb.2019.00841, 10
Abe F, Muto M, Yaeshima T, Iwatsuki K, Aihara H, Ohashi Y, Fujisawa T (2010) Safety evaluation of probiotic bifidobacteria by analysis of mucin degradation activity and translocation ability. Anaerobe 16(2):131–136. https://doi.org/10.1016/j.anaerobe.2009.07.006
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 240 kb)
Rights and permissions
About this article
Cite this article
Rastogi, S., Mittal, V. & Singh, A. Selection of Potential Probiotic Bacteria from Exclusively Breastfed Infant Faeces with Antagonistic Activity Against Multidrug-Resistant ESKAPE Pathogens. Probiotics & Antimicro. Prot. 13, 739–750 (2021). https://doi.org/10.1007/s12602-020-09724-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-020-09724-w