Abstract
A species of Heterodera has been known to parasitise cereals in Australia since the 1930s. It caused significant yield losses across Australia’s cereal growing regions until resistance breeding largely brought it under control, although it still occurs occasionally, especially in South and Western Australia. Australian cereal cyst nematode has long been considered to represent Heterodera avenae. However, in 2002 the name Heterodera australis was proposed for Australian cereal cyst nematode, as it could be distinguished from all non-Australian populations of H. avenae via biochemical and molecular methods. This new species proposal came with speculation that both H. avenae and H. australis might occur in Australia, and that H. australis might represent a native species. The name H. australis has generally not been accepted by Australian scientists, nor the notion that it is native. There remains some uncertainty as to the validity of H. australis and whether more than one species of cereal cyst nematode occur in Australia. Using a molecular barcoding approach (COI, 18S, ITS, 28S) we examined the species composition of cyst nematodes present in soil samples collected between 1989–2023 from Australian cereal growing regions. We find only one species of Heterodera parasitising cereals and, based on phylogenetic analyses, accept the validity of H. australis as the name best representative of this species. We also argue that, based on presently available evidence, H. australis is not native and was most likely introduced into Australia from Asia in the 1850s, rather than from Europe as has been generally assumed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first reports of a species of Heterodera parasitising cereals in Australia were made in the 1930s. The species in question was then considered to represent the ‘oat strain’ of Heterodera schachtii A. Schmidt, 1871 (Davidson, 1930; Hickenbotham, 1930; Millikan, 1938). Franklin (1940) later designated the ‘oat strain’ of H. schachtii as Heterodera major O. Schmidt, 1930, but the name Heterodera avenae Wollenweber, 1924 was subsequently adopted because of taxonomic priority (Franklin et al., 1959). By the 1980s Australian cereal cyst nematode (CCN) was known from New South Wales, South Australia, Victoria, and Western Australia, with yield losses in South Australia and Victoria alone estimated at upwards of $72 million AUD per annum (Brown, 1984; McLeod et al., 1986; Meagher, 1968; Parkin & Goss, 1968). Development of resistant cereal varieties have since led to highly successful and broad scale control of CCN in Australia, with population densities and distributions of this nematode in significant decline (Lewis et al., 2009; Ogbonnaya et al., 2009; Riley & McKay, 2009; Vanstone et al., 2008). By 2009, CCN retained a patchy distribution in South and Western Australia, but had become rare in Victoria and was no longer being detected in New South Wales (Riley & McKay, 2009). Multiple reviews of research on Australian CCN are available (Lewis et al., 2009; Ogbonnaya et al., 2009; Riley & McKay, 2009; Stirling et al., 2008; Vanstone et al., 2008), thus readers interested in the historic perspective, local ecology of Australian CCN and background for resistance breeding are pointed to these works for further details.
A number of early papers suggested that Australian CCN might represent a species distinct from European H. avenae (e.g., Thorne, 1961; Wallace, 1965; Webster, 1969; Winslow, 1960). Additional work demonstrated that Australian CCN populations represented a single pathotype which was distinct from those known in Europe and elsewhere (Andersen & Andersen, 1982; Brown, 1969, 1982; Brown & Meagher, 1970; O'Brien & Fisher, 1979). However, after morphological comparison of Australian, European, and Canadian cereal cyst nematodes, Meagher (1974) concluded that there was no justification for considering Australian CCN as a species distinct from H. avenae. This conclusion was later supported through further morphological studies by McLeod and Khair (1977).
Whether Australian CCN represented a species distinct from H. avenae was again brought to question by Rumpenhorst (1988) via isoelectric focusing of proteins from Australian, European, and Israeli isolates of nematodes identified as H. avenae. Rumpenhorst (1988) noted that the protein pattern observed from Australian isolates differed significantly from those from Europe and Israel and suggested this could be interpreted as a species-level difference. Ferris et al., (1994), employing two-dimensional gel electrophoresis (2-DGE) and sequencing of the nuclear ribosomal internal transcribed spacer (ITS) gene region, also noted a difference in protein patterns and ITS sequences between Australian and European H. avenae. In contrast, Bossis and Rivoal (1996) considered the protein patterns of Australian and French H. avenae as observed via 2-DGE to be similar and Bekal et al., (1997) observed that Australian H. avenae showed the same ITS restriction fragment length polymorphism (RFLP) profile as populations from elsewhere. Ultimately, after comparison of Australian and other global populations of putative H. avenae via morphology, protein patterns, RFLP-ITS profiles, and phylogenetic analysis of the ITS gene region, Subbotin et al., (2002) chose to describe the Australian cereal cyst nematode as a new species, Heterodera australis Subbotin, Rumpenhorst, Sturhan & Moens, 2002, based on material sourced from South Australia and Victoria.
The original proposal of H. australis by Subbotin et al., (2002) has not, for the most part, been accepted by Australian researchers (Riley & McKay, 2009; Vanstone et al., 2008). Criticisms of the original proposal of H. australis include the lack of any observable morphological differences between H. australis and H. avenae, the lack of any host or clear ecological differences, and that biochemical and molecular differences in themselves do not necessarily warrant distinct species status (Riley & McKay, 2009; Vanstone et al., 2008). In particular, Australian researchers have objected to the suggestion of Subbotin et al., (2002) that there may be two species of cereal cyst nematode occurring in Australia, one introduced, and one native (Riley & McKay, 2009; Vanstone et al., 2008). Australian researchers have been of the opinion that the Australian CCN represents one non-native species derived from a single introduction because there is limited ecological and genetic diversity within populations, no differing genotypic reactions in cereals and no known native hosts (Brown, 1982, 1984; McLeod, 1992; Riley & McKay, 2009; Stanton & Eyres, 1994; Vanstone et al., 2008). Despite a number of subsequent studies incorporating new molecular data and phylogenetic methods supporting a distinction between H. australis and H. avenae (Subbotin, 2015; Subbotin et al., 2003, 2018), H. avenae is still the primary name in use in Australia. Thus, there remains a level of uncertainty as to whether one or more species of CCN occur in Australia, where these putatively different species may occur and how they can be diagnosed.
Here, we survey Australian CCN populations using a DNA-barcoding approach. We provide novel genetic and morphological data generated from cyst nematodes extracted from archival and newly collected samples from multiple localities across Australia’s grain growing regions, discuss the taxonomic nomenclature of Australian CCN and speculate on the probable timing and route of its introduction into Australia.
Methods
Specimen collection and morphological study
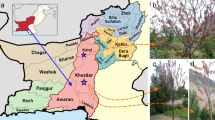
Heteroderid cysts were extracted from soil samples collected from across grain growing regions of south-eastern and Western Australia between the late 1980s and 2023 (Fig. 1; Table 1) via Cobb’s sieving and decanting method (Cobb, 1918). Sampling between 2020 and 2023 targeted areas where commercial testing had previously indicated CCN presence. Vulval cone specimens were produced by placing cysts in a drop of water, cutting them in half with a scalpel blade and scraping the interior contents out carefully using fine dissecting forceps and needles followed by mounting on slides in a modified Kaiser’s glycerin jelly (see Dioni, 2003). J2 larva were obtained from cysts concurrently with vulval cone mounting, from additional crushed cysts, or as free individuals which had emerged from cysts which had been soaked in water at 3–4 °C for several days to several months. Some J2 larvae were studied live using temporary wet mounts and others were killed in near-boiling water, preserved in 4% formalin, processed to glycerol using the slow method (Hooper, 1986) and mounted in glycerol on wax-ring slides. Male heteroderids were obtained by placing 10 cysts into 9 mm pots containing two-week old wheat seedlings growing in commercial seed-raising soil under fluorescent lights at the CSIRO Black Mountain site in Canberra, Australian Capital Territory. After three months, nematodes were extracted from the inoculated soil using Whitehead-Hemming trays (Whitehead & Hemming, 1965). Male heteroderids were collected, studied live, killed in near-boiling water, and processed to wax-ring glycerol slides as above. Photographs and measurements of specimens were taken using a ZEISS Axiocam 506 mono camera mounted on a ZEISS Axioscope light microscope and associated ZEISS Blue imaging software (ZEISS, Germany). Images were edited and annotated in Adobe Illustrator CS6. Voucher specimens of vulval cones, J2s and males are lodged in the Nematology section of the Australian National Insect Collection, Canberra, Australia, under the accession numbers 8866–8905.
Molecular and phylogenetic study
Collection sites were screened for heteroderid species composition through extraction of DNA from crushed whole cysts and/or from J2 larva and eggs collected from cysts. Genomic DNA was extracted from specimens using DNeasy Blood and Tissue kits (Qiagen®) following the manufacturer’s instructions. Four molecular markers were targeted: the mitochondrial cytochrome c oxidase I (COI) gene region, the small subunit ribosomal RNA (18S rRNA), the internal transcribed spacer region (ITS; comprising ITS1-5.8S-ITS2) and the large subunit ribosomal RNA (28S rRNA). The COI region was used as the primary barcode for surveying collection sites as this marker has been shown to reliably differentiate cereal cyst nematodes (Huston et al., 2022; Subbotin et al., 2018). The COI region was amplified using the forward primer JB3 (5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) (Bowles et al., 1992) and reverse JB5 (5′-AGC ACC TAA ACT TAA AAC ATA ATG AAA ATG-3′) (Derycke et al., 2005). The 18S region was amplified in two fragments, with the first fragment using the forward primer G18S4 (5′-GCT TGT CTC AAA GAT TAA GCC-3′) (Blaxter et al., 1998) and reverse R18Ty11 (5′-GGT CCA AGA ATT TCA CCT CTC-3′) (Chizhov et al., 2006) and the second fragment using the forward primer F18Ty12 (5′-CAG CCG CGG TAA TTC CAG C-3′) (Chizhov et al., 2006) and reverse R18Ty12 (5′-CGG TGT GTA CAA AGG GCA GG-3′) (Chizhov et al., 2006). The ITS region was amplified using the forward primer TW81 (5′-GTT TCC GTA GGT GAA CCT GC-3′) and reverse AB28 (5′-ATA TGC TTA AGT TCA GCG GGT-3′) (Curran et al., 1994). The 28S region was amplified using the forward primer D2A (5′-ACA AGT ACC GTG AGG GAA AGT TG-3′) and reverse primer D2B (5′-TCG GAA GGA ACC AGC TAC TA′) (Nunn, 1992). PCR and clean-up followed Huston et al., (2023b) and was the same for all four gene regions. PCR products were sent to the Biomolecular Resource Facility, Australian National University, Canberra, for Sanger sequencing and sequenced using the amplification primers. Resultant reads were assembled and edited using Geneious Prime® v2022.1.1 (Biomatters).
Newly generated sequences were preliminarily identified to species through comparison against the NCBI GenBank database via BLAST (Altschul et al., 1990). Intraspecific variation among newly generated sequences was evaluated through alignment using MUSCLE (Edgar, 2004) as implemented in MEGA11 (Tamura et al., 2021). Newly generated sequences representing various geographic localities and all unique variants detected for each gene region were aligned with those of other members of the Heterodera avenae species group using MUSCLE and MEGA11 as above. Interspecific variation between newly generated sequence and those of the Heterodera avenae species group were further examined using pairwise comparison tables, and best-fit nucleotide substitution models for phylogenetic analyses were evaluated for the COI, ITS and 28S datasets, using MEGA11. The HKY + I + G, GTR + G and HKY + G substitution models were selected for the COI, ITS and 28S datasets, respectively. Phylogenetic analyses were not performed for the 18S gene region because this gene has been shown to be highly conserved within the genus Heterodera and to lack utility for within-genus phylogenetic inference for some groups (Huston et al., 2022). Phylogenetic analyses were performed on XSEDE (Towns et al., 2014) accessed through the CIPRES portal (Miller et al., 2010), using sequences of Heterodera glycines and Heterodera schachtii as outgroup taxa. Majority-rule consensus trees were constructed using Bayesian inference (BI) and maximum likelihood (ML) analyses. Bayesian inference was performed using MrBayes v3.2.6 (Ronquist et al., 2012) with default priors and four chains sampled every 1,000 of 10,000,000 generations; the first 2,500 samples were discarded as burn-in. Maximum likelihood analyses were performed using RAxML (Stamatakis, 2014) with 1,000 bootstrap pseudoreplicates. Combined BI/ML trees were edited and annotated in Adobe Illustrator CS6.
Results
Heteroderid cysts were recovered from 17 South Australian localities (comprising 21 paddocks), one Victorian locality (one paddock) and 10 Western Australian localities (15 paddocks) (Fig. 1; Table 1). Additionally, cysts were obtained from a culture long held by SARDI, and cysts from this culture were those used to produce male nematodes in the pot trials described above.
We generated 128, 11, 17, and 20 sequences of the COI, 18S, ITS, and 28S gene-regions, respectively, from across our collection localities, from the SARDI culture and from males from our own pot trials (Table 1). With the exception of three localities where other cyst nematodes were detected (see below), species assignments based on BLAST of COI sequences demonstrated all populations represented H. australis (Table 1). No intraspecific variation was detected among the 119 COI sequences of H. australis generated; three voucher sequences were submitted to GenBank, one from each Australian state (OR701393–OR701395). Sequences of the 18S region comprised two variants differing by one single nucleotide polymorphism (SNP); two voucher sequences were submitted to GenBank (OR701403–OR701404). Sequences of the ITS region comprised four variants, differing by up to four SNPs; five voucher sequences were submitted to GenBank (OR701388–OR701392). Sequences of the 28S comprised two variants based on two SNPs; four voucher sequences were submitted to GenBank (OR701398–OR701401).
Heterodera schachtii was detected at two localities in South Australia; this species has been reported widely throughout Australia (McLeod et al., 1994). Sequences of the COI and 28S gene generated for H. schachtii lacked intraspecific variation and voucher sequences are submitted under the GenBank accessions OR701397 (COI) and OR701402 (28S). Heterodera mani was detected at one locality in Western Australia in the present study; this species was recently reported for the first time in Western Australia from a different locality (Huston et al., 2023a) and has also been reported from Tasmania (Jain et al., 2023b). One sequence of the COI gene for H. mani was submitted to GenBank (OR701396). No additional cyst nematode species were detected in any of the samples from this study.
Bayesian and maximum likelihood analyses of the COI dataset produced congruent topologies (Fig. 2). The newly generated sequences of H. australis resolved with strong support in a clade with previously available sequences of H. australis provided by Subbotin et al., (2018). Although Heterodera aucklandica, Heterodera arenaria + H. avenae, and Heterodera pratensis + Heterodera sturhani all fell within well-supported clades, all these clades plus the H. australis clade resolved in a large polytomy, sister to Heterodera ustinovi. Bayesian and maximum likelihood analyses of the ITS dataset (Fig. 3) also produced largely congruent topologies, but with a smaller crown polytomy including two supported monophyletic clades, one comprising sequences of H. australis from Australia and China, and the other comprising sequences of H. pratensis and H. sturhani, and a number of additional sequences of H. australis from Australia and China on independent branches. Tree topologies from Bayesian and maximum likelihood analyses of the 28S dataset (Fig. 4) were largely congruent due to the lack of support for most clades. The only clades supported as monophyletic were those of Heterodera latipons and Heterodera filipjevi; all other sequences could not be phylogenetically differentiated thus falling in a large polytomy of independent branches.
Bayesian majority-rule consensus tree of the COI mtDNA dataset. Bayesian inference (BI) posterior probabilities (pp) for nodes represented by circles, maximum likelihood (ML) bootstrap support (bs) represented by squares. Support values less than 0.90 (pp) and 70 (bs) not shown. The scale-bar indicates the number of substitutions per site. GenBank accession number presented after taxa name
Bayesian majority-rule consensus tree of the ITS rDNA dataset. Bayesian inference (BI) posterior probabilities (pp) for nodes represented by circles, maximum likelihood (ML) bootstrap support (bs) represented by squares. Support values less than 0.90 (pp) and 70 (bs) not shown. The scale-bar indicates the number of substitutions per site. GenBank accession number presented after taxa name
Bayesian majority-rule consensus tree of the 28S rDNA dataset. Bayesian inference (BI) posterior probabilities (pp) for nodes represented by circles, maximum likelihood (ML) bootstrap support (bs) represented by squares. Support values less than 0.90 (pp) and 70 (bs) not shown. The scale-bar indicates the number of substitutions per site. GenBank accession number presented after taxa name
Qualitative morphological and morphometric data (Fig. 5, 6; Table 2) of all specimens from populations identified as H. australis via molecular barcoding were consistent with the concept of H. australis as described by Subbotin et al., (2002). No further differences were found between H. australis and H. avenae despite several additional measurements and indices being taken (see Table 2); thus, these two species remain morphologically indistinguishable.
Vulval cones of Heterodera australis. a, surface view of vulval plate showing fenestrae. b, surface view of vulval plate showing fenestrae, vulval slit indicated by arrow. c, surface view of vulval plate showing fenestrae, anus indicated by arrow. d, Lateral view of vulval cone showing strongly-developed bullae. Scale bars = 20 µm
Discussion
Based on the number of sequences generated and the breadth of cyst nematode populations studied, we conclude that H. australis is very likely the only cyst nematode impacting cereals in Australia, and notably, H. avenae appears absent from the continent. This has some biosecurity implications because H. avenae is generally considered the species of cereal cyst nematode present in Australia, and the Australian biosecurity community has not universally accepted that H. avenae and H. australis represent different species (Riley & McKay, 2009; Vanstone et al., 2008). For example, Heterodera carotae, Heterodera filipjevi, Heterodera glycines, Heterodera latipons, Heterodera sorghi, and Heterodera zeae all appear on Australia’s current (2019) list of National Priority Plant Pests, but H. avenae does not. Despite this lack of nomenclatural clarity, however, it has long been recognised that Australian CCN populations comprise a single pathotype (Ha13) distinct from the many other H. avenae pathotypes occurring elsewhere (Andersen & Andersen, 1982; Brown, 1982; O'Brien & Fisher, 1979; Rivoal & Cook, 1993; Vanstone et al., 2008).
With no known morphological or host differences, H. australis and H. avenae are distinguished from one another only through differences in pathogenicity, biochemical signatures, and molecular sequence data (Subbotin, 2015; Subbotin et al., 2002, 2018). It is possible that, rather than truly representing species-level differences, this genetic and biochemical divergence is simply representative of the eastern and western extremes of natural intraspecific variation present in a widespread species. Although H. australis represents a unique pathotype among cereal cyst nematodes and there seems some evidence of species/pathotype relationships (Subbotin et al., 2018), there are many pathotypes presently comprising H. avenae sensu stricto (Andersen & Andersen, 1982; Rivoal & Cook, 1993; Subbotin et al., 2018; Vanstone et al., 2008). Thus, there are clear issues with using a ‘molecular yardstick’ approach for species delineation within the H. avenae species group. However, in this case, the positions of other members of the H. avenae group within the topologies of the present phylogenetic analyses are potentially informative regarding the species status of H. australis.
Although analyses of the 28S gene region did not distinguish between most members of the H. avenae group, analyses of the ITS and COI genes demonstrate that H. australis and H. avenae are not each other’s closest relatives. Heterodera aucklandica and H. mani are either more closely related to H. avenae or equally related to both H. avenae and H. australis and have seemingly legitimate host and morphological differences (Subbotin et al., 2010) that distinguish them from H. avenae and H. australis. Similarly, in the present ITS and COI analyses, H. pratensis + H. sturhani are either more closely related to H. australis, or equally related to both H. australis and H. avenae. Heterodera pratensis is generally not thought to parasitise cereals (but see Qing et al., 2022) and males have spicules with a tridentate terminus, rather than bifid as in H. avenae and H. australis (Subbotin et al., 2010). Heterodera sturhani is a parasite of cereals, and its morphometrics overlap with those of H. australis and H. avenae (Subbotin, 2015; Subbotin et al., 2010). A male has not been reported for H. sturhani (Subbotin, 2015). However, because of its sister relationship between H. pratensis and H. sturhani in the present COI and ITS analyses, plus the findings of Qing et al., (2022) which suggest the two species may be conspecific, it seems reasonable to speculate that males of H. sturhani will have spicules with a tridentate terminus.
Based on the above, we think that there is some justification for recognising H. australis as a species distinct from H. avenae. Synonymizing H. australis with H. avenae seems of little practical value at present and to do so would suggest that a number of other species within the H. avenae species group should also be synonymized. In the case of H. australis at least, this could negatively impact national biosecurity systems, considering the former species name is representative of a distinct pathotype.
The prevailing hypothesis regarding the origin of CCN in Australia is that the species was introduced from Europe via contaminated farm equipment sometime in the 1800s (McLeod, 1992; Meagher, 1977; Riley & McKay, 2009; Vanstone et al., 2008). In the original description of H. australis, Subbotin et al., (2002) also suggested that the species might be native to Australia, however, H. australis has never been found occurring on native Australian plants (McLeod, 1992; McLeod et al., 1994). Subsequent molecular studies have provided data indicating that H. australis is present in China (Fu et al., 2011; Ou et al., 2008; Shao et al., 2023; Subbotin et al., 2018; Yuan et al., 2010) and researchers have suggested Australian and Chinese CCN populations may have a common origin (Shao et al., 2022, 2023; Subbotin et al., 2018). During the Australian goldrush of the 1850s, between 40,000–50,000 Chinese migrants arrived in Australia, primarily to Victoria and New South Wales (Frost, 2002; Reeves & Mountford, 2011). Most of these migrants were from the Pearl River delta of southern China (Reeves & Mountford, 2011), where traditional farmers generally produced two crops of rice and one of either wheat or vegetables per year (Marks, 1996). It seems likely that Chinese migrants heading to the goldfields of Victoria and New South Wales would bring with them tools for digging, mining, and gardening, thus providing a simple means for the unintentional introduction of soil-borne pests such as H. australis. Based on herbarium specimens, H. australis has been present in Australia since at least 1904 (Meagher, 1977), which fits reasonably well with an introduction during the 1850s. Furthermore, H. australis has not been detected in Europe or western Asia, and genetic divergence between H. australis and European populations of H. avenae is significant. This can be contrasted with the seemingly likely introduction of Heterodera humuli into Australia from England in 1822; even after 200 years Australian populations of H. humuli have barely diverged genetically from those in Europe (Jain et al., 2023a). Therefore, available evidence indicates that H. australis is not native to the Australian continent and, rather than being introduced from Europe, it was most likely first introduced into Victoria or New South Wales from China in the 1850s, with subsequent spread into South and Western Australia.
The centre of origin of wheat and barley agriculture is generally thought to be the fertile crescent in southwestern Asia with several varieties of wheat and barley being domesticated around 8500–7500 BCE (Betts et al., 2014; Zohary et al., 2012). The centre of origin of most species of the Heterodera avenae group appears to correspond with the centre of origin of wheat and barley (Subbotin et al., 2018), although recent evidence suggests northwest China may also be a centre of cereal cyst nematode diversification (Qing et al., 2022; Shao et al., 2022, 2023), so it is possible that H. australis originated there. There is ample evidence of the ability of heteroderids to disperse widely without human assistance, e.g., via wind (Meagher, 1982; White, 1953), but as with cereals, human-mediated dispersal has undoubtably had a significant impact on the distribution of these nematodes (Qing et al., 2022; Shao et al., 2022).
Subbotin et al., (2018) argued that taxonomic differentiation among the H. avenae group is needed for biosecurity regulation and that species that infest cereals should be subject to phytosanitary action, whereas species such as H. arenaria and H. pratensis have little economic relevance. We would like to highlight that in the same publication Subbotin et al., (2018) speculated that H. arenaria had recently diverged from European cereal-parasitising H. avenae into coastal grasses, and that H. sturhani had recently diverged from the non-cereal grass parasite H. pratensis in East Asia via acquisition of the ability to parasitise cereals. This would suggest that members of the H. avenae group exhibit enough plasticity in their host requirements to be able to host-switch from non-cereal grasses to cereals and vice versa. If H. sturhani is truly recently diverged from H. pratensis, then efforts to prevent the establishment of the latter species in new localities should also be made, as this species may be able host-switch into cereals again. We also note that in a recent study, Qing et al., (2022) reported populations of cyst nematodes corresponding to variants intermediate between H. pratensis and H. sturhani occurring in China which can parasitise both cereal and non-cereal grasses, suggesting these two species are conspecific. Heterodera arenaria may retain the genes needed to host switch from coastal grasses back into cereals. Thus, all members of H. avenae group should be considered of biosecurity concern regardless of their taxonomic status, as there is no reason to assume that parasites which have completed a host switch once will not do so again.
Conclusions
We conclude that Heterodera australis is an acceptable taxonomic name for the Australian cereal cyst nematode and that this species is the only one parasitising cereals in Australia at present. We also conclude that H. australis is not native to Australia and was most likely introduced from China in the 1850s, rather than from Europe as has been previously assumed.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CCN:
-
Cereal cyst nematode
- COI:
-
Mitochondrial cytochrome c oxidase I
- 18S:
-
Small subunit ribosomal RNA
- ITS:
-
Internal transcribed spacer region
- 28S:
-
Large subunit ribosomal RNA
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Andersen, S., & Andersen, K. (1982). Suggestions for determination and terminology of pathotypes and genes for resistance in cyst-forming nematodes, especially Heterodera avenae. EPPO Bulletin, 12, 379–386. https://doi.org/10.1111/j.1365-2338.1982.tb01819.x
Bekal, S., Gauthier, J. P., & Rivoal, R. (1997). Genetic diversity among a complex of cereal cyst nematodes inferred from RFLP analysis of the ribosomal internal transcribed spacer region. Genome, 40, 479–486. https://doi.org/10.1139/g97-064
Betts, A., Jia, P. W., & Dodson, J. (2014). The origins of wheat in China and potential pathways for its introduction: A review. Quaternary International, 348, 158–168. https://doi.org/10.1016/j.quaint.2013.07.044
Blaxter, M. L., De Ley, P., Garey, J. R., Liu, L. X., Scheldeman, P., Vierstraete, A., Vanfleteren, J. R., Mackey, L. Y., Dorris, M., & Frisse, L. M. (1998). A molecular evolutionary framework for the phylum Nematoda. Nature, 392, 71–75. https://doi.org/10.1038/32160
Bossis, M., & Rivoal, R. (1996). Protein variability in cereal cyst nematodes from different geographic regions assessed by two-dimensional gel electrophoresis. Fundamental and Applied Nematology, 19, 25–34.
Bowles, J., Blair, D., & McManus, D. P. (1992). Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Molecular and Biochemical Parasitology, 54, 165–173. https://doi.org/10.1016/0166-6851(92)90109-W
Brown, R. H. (1969). The occurrence of biotypes of the cereal cyst nematode (Heterodera avenae Woll.) in Victoria. Australian Journal of Experimental Agriculture, 9, 453–456. https://doi.org/10.1071/EA9690453
Brown, R. H. (1982). Studies on the Australian pathotype of Heterodera avenae. EPPO Bulletin, 12, 413–416. https://doi.org/10.1111/j.1365-2338.1982.tb01823.x
Brown, R. H. (1984). Ecology and control of cereal cyst nematode (Heterodera avenae) in southern Australia. Journal of Nematology, 16, 216–222.
Brown, R. H., & Meagher, J. W. (1970). Resistance in cereals to the cyst nematode (Heterodera avenae) in Victoria. Australian Journal of Experimental Agriculture, 10, 360–365. https://doi.org/10.1071/EA9700360
Chizhov, V. N., Chumakova, O. A., Subbotin, S. A., & Baldwin, J. G. (2006). Morphological and molecular characterization of foliar nematodes of the genus Aphelenchoides: A. fragariae and A. ritzemabosi (Nematoda: Aphelenchoididae) from the Main Botanical Garden of the Russian Academy of Sciences. Russian Journal of Nematology, 14, 179–184.
Cobb, N. A. (1918). Estimating the nema population of soil with special references to the sugarbeet and root-gall nemas, Heterodera schachtii Schmidt and Heterodera radicicola (Greef) Muller, and with a description of Tylencholaimus aequalis n. sp. United States Department of Agriculture, Agricultural Technology Circular 1.
Curran, J., Driver, F., Ballard, J. W. O., & Milner, R. J. (1994). Phylogeny of Metarhizium: Analysis of ribosomal DNA sequence data. Mycological Research, 98, 547–552. https://doi.org/10.1016/S0953-7562(09)80478-4
Davidson, J. (1930). Eelworms (Heterodera schachtii) affecting cereals in South Australia. Journal of Department of Agriculture, South Australia, 34, 378–385.
Derycke, S., Remerie, T., Vierstraete, A., Backeljau, T., Vanfleteren, J., Vincx, M., & Moens, T. (2005). Mitochondrial DNA variation and cryptic speciation within the free-living marine nematode Pellioditis marina. Marine Ecology Progress Series, 300, 91–103. https://doi.org/10.3354/meps300091
Dioni, W. (2003). Safe microscopical reagents for amateurs. Mounting microscopic subjects. Part 4-Glycerine jellies. Part 5-Mountant summary. Micscape Magazine. Microscopy UK. http://www.microscopy-uk.org.uk/mag/artaug03/wdpart4.html.
Edgar, R. C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797. https://doi.org/10.1093/nar/gkh340
Ferris, V. R., Ferris, J. M., Faghihi, J., & Ireholm, A. (1994). Comparisons of isolates of Heterodera avenae using 2-D PAGE protein patterns and ribosomal DNA. Journal of Nematology, 26, 144–151.
Franklin, M. T. (1940). On the specific status of the so-called biological strains of Heterodera schachtii Schmidt. Journal of Helminthology, 18, 193–207. https://doi.org/10.1017/S0022149X00031515
Franklin, M. T., Thorne, G., & Oostenbrink, M. (1959). Proposal to stabilise the scientific name of the cereal-root eelworm (Class Nematoda). Bulletin of Zoological Nomenclature, 17, 76–85.
Frost, W. (2002). Migrants and technological transfer: Chinese farming in Australia, 1850–1920. Australian Economic History Review, 42, 113–131. https://doi.org/10.1111/1467-8446.t01-1-00007
Fu, B., Yuan, H.-X., Zhang, Y., Hou, X.-S., Nian, G.-L., Zhang, P., Xing, X.-P., Sun, B.-J., Riley, I. T., & Li, H.-L. (2011). Molecular characterisation of cereal cyst nematodes in winter wheat on the Huang-Huai floodplain of China using RFLP and rDNA-ITS sequence analyses. Australasian Plant Pathology, 40, 277–285. https://doi.org/10.1007/s13313-011-0043-0
Hickenbotham, A. R. (1930). Eelworm and "no growth" patches. Journal of the Department of Agriculture, South Australia, 34, 386–392.
Hooper, D. J. (1986). Handling, fixing, staining and mounting nematodes. In Southey, J.F. (Ed.), Laboratory methods for work with plant and soil nematodes (pp. 59–80). Her Majesty’s Stationery Office.
Huston, D. C., Khudhir, M., & Hodda, M. (2022). Reliability and utility of standard gene sequence barcodes for the identification and differentiation of cyst nematodes of the genus Heterodera. Journal of Nematology, 54, 1–24. https://doi.org/10.2478/jofnem-2022-0024
Huston, D. C., Hodda, M., Hills, A., & Collins, S. (2023a). Detection of Heterodera mani in Western Australia. Australasian Plant Disease Notes, 18, 1–7. https://doi.org/10.1007/s13314-023-00503-4
Huston, D. C., Khudhir, M., & Hodda, M. (2023b). Phylogenetic position of Ptychaphelenchus eucalypticola Hodda, 2009 within the Aphelenchoidoidea Skarbilovich, 1947 (Siddiqi, 1980) inferred from partial 18S and 28S rDNA gene sequences. Nematology, 25, 59–76. https://doi.org/10.1163/15685411-bja10206
Jain, A., Huston, D. C., Wainer, J., Hodda, M., Hayes, O., Whittock, S., Darling, E., Mann, R., Edwards, J., & Rodoni, B. (2023a). Geographic range extension of hop cyst nematode, Heterodera humuli, from Tasmania to the Australian mainland. Australasian Plant Disease Notes, 18, 1–8. https://doi.org/10.1007/s13314-023-00494-2
Jain, A., Wainer, J., Huston, D. C., Hodda, M., Dinh, Q., Mann, R., Rodoni, B., & Edwards, J. (2023b). First report of ryegrass cyst nematode, Heterodera mani, in Tasmania, Australia. Plant Disease, 107, 1245. https://doi.org/10.1094/PDIS-05-22-1129-PDN
Lewis, J. G., Matic, M., McKay, A. C. (2009). Success of cereal cyst nematode resistance in Australia: history and status of resistance screening systems. In Riley I. T., Nicol J. M., Dababat A. A. (Eds.), Cereal cyst nematodes: status, research and outlook. Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative, Antayla, Turkey, (pp. 137–142). International Maize and Wheat Improvement Centre (CIMMYT).
Marks, R. B. (1996). Commercialization without capitalism: Processes of environmental change in South China, 1550–1850. Environmental History, 1, 56–82.
McLeod, R. W. (1992). On the origin and spread of Heterodera avenae in Australia. Nematologia Mediterranea, 20, 59–61.
McLeod, R. W., & Khair, G. T. (1977). Observations on the morphology of Heterodera avenae in Australia. Nematologica, 23, 382–389.
McLeod, R. W., Wong, P. T. W., & Southwell, R. J. (1986). Biology and control of cereal cyst nematode in northern New South Wales. Australian Journal of Experimental Agriculture, 26, 375–381. https://doi.org/10.1071/EA9860375
McLeod, R., Reay, F., & Smyth, J. (1994). Plant nematodes of Australia listed by plant and by genus. NSW Department of Agriculture.
Meagher, J. W. (1968). The distribution of the cereal cyst nematode (Heterodera avenae) in Victoria and its relation to soil type. Australian Journal of Experimental Agriculture, 8, 637–640. https://doi.org/10.1071/EA9680637
Meagher, J. W. (1974). The morphology of the cereal cyst nematode (Heterodera avenae) in Australia. Nematologica, 20, 1–8. https://doi.org/10.1163/187529274X00519
Meagher, J. W. (1977). World dissemination of the cereal-cyst nematode (Heterodera avenae) and its potential as a pathogen of wheat. Journal of Nematology, 9, 9–15.
Meagher, J. W. (1982). The effect of environment on survival and hatching of Heterodera avenae. EPPO Bulletin, 12, 361–369. https://doi.org/10.1111/j.1365-2338.1982.tb01817.x
Miller, M. A., Pfeiffer, W., Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE) New Orleans, LA, p. 1–8.
Millikan, C. R. (1938). Eelworm (Heterodera schachtii Schmidt) disease of cereals. Journal of the Department of Agriculture, Victoria, 36, 452–468.
Nunn, G. B. (1992). Nematode molecular evolution: an investigation of evolutionary patterns among nematodes based upon DNA sequences. Ph. D. Thesis, University of Nottingham, Nottingham, UK.
O’Brien, P. C., & Fisher, J. M. (1979). Reactions of cereals to populations of Heterodera avenae in South Australia. Nematologica, 25, 261–267. https://doi.org/10.1163/187529279X00280
Ogbonnaya, F. C., Eastwood, R. F., Lagudah, E. (2009). Identification and utilisation of genes for cereal cyst nematode resistance (Heterodera avenae) resistance in wheat: the Australian experience. In Riley I. T., Nicol J. M., Dababat A. A. (Eds.), Cereal cyst nematodes: status, research and outlook. Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative, Antayla, Turkey (pp. 166–171). International Maize and Wheat Improvement Centre (CIMMYT).
Ou, S. Q., Peng, D. L., Li, Y., & Wang, Y. J. (2008). Restriction fragment length polymorphism and sequences analysis of rDNA-ITS region of cereal cyst nematode (Heterodera avenae) on wheat from Zhengzhou. Acta Phytopathologica Sinica, 38, 407–413.
Parkin, R. J., Goss, O. M. (1968). Cereal eelworm: a new disease of cereal crops in the Geraldton area. Journal of the Department of Agriculture, Western Australia, Series 4, 9, 116–120.
Qing, X., Peng, H., Ma, J., Zhang, Y. M., Li, H., Peng, D., Wang, X., & Long, T. (2022). Phylogeography of Chinese cereal cyst nematodes sheds lights on their origin and dispersal. Evolutionary Applications, 15, 1236–1248. https://doi.org/10.1111/eva.13452
Reeves, K., & Mountford, B. (2011). Sojourning and settling: Locating Chinese Australian history. Australian Historical Studies, 42, 111–125. https://doi.org/10.1080/1031461X.2010.539620
Riley, I. T., McKay, A. C. (2009). Cereal cyst nematode in Australia: biography of a biological invader. In Riley I. T., Nicol J. M., Dababat A. A. (Eds.), Cereal cyst nematodes: status, research and outlook. Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative, Antayla, Turkey (pp. 23–28). International Maize and Wheat Improvement Centre (CIMMYT).
Rivoal, R., & Cook, R. (1993). Nematode pests of cereals. In K. Evans, D. L. Trudgill, & J. M. Webster (Eds.), Plant Parasitic Nematodes in Temperate Agriculture (pp. 259–303). Wallingford.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M. A., & Huelsenbeck, J. P. (2012). MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542. https://doi.org/10.1093/sysbio/sys029
Rumpenhorst, H. J. (1988). Protein taxonomic studies on Heterodera avenae and related species. Biologische Bundesanstalt Für Land Und Forstwirtschaft Berlin Und Braunschweig, Jahresbericht, 1987, 67.
Shao, H., Xue, Q., Yao, K., Cui, J., Huang, W., Kong, L., Li, C., Li, H., Peng, D., & Smiley, R. W. (2022). Origin and phylogeography of Chinese cereal cyst nematode Heterodera avenae revealed by mitochondrial COI sequences. Phytopathology, 112, 1988–1997. https://doi.org/10.1094/PHYTO-12-21-0532-R
Shao, H., Zhu, L., Li, Z., Jiang, R., Liu, S., Huang, W., Li, C., Kong, L.-A., Peng, D., & Peng, H. (2023). Population genetics of the cereal cyst nematode Heterodera avenae reveal geographical segregation and host adaptation. Phytopathology Research, 5, 30. https://doi.org/10.1186/s42483-023-00185-x
Stamatakis, A. (2014). RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Stanton, J. M., & Eyres, M. (1994). Hatching of Western Australian populations of cereal cyst nematode, Heterodera avenae, and effects of sowing time and method of sowing on yield of wheat. Australasian Plant Pathology, 23, 1–7. https://doi.org/10.1071/APP9940001
Stirling, G. R., Yeates, G. W., Davies, K., & Hodda, M. (2008). The history of plant and soil nematology in Australia and New Zealand, with particular reference to the contributions of six pioneering nematologists. Australasian Plant Pathology, 37, 203–219. https://doi.org/10.1071/AP08017
Subbotin, S. A. (2015). Heterodera sturhani sp. n. from China, a new species of the Heterodera avenae species complex (Tylenchida: Heteroderidae). Russian Journal of Nematology, 23, 145–152.
Subbotin, S. A., Sturhan, D., Rumpenhorst, H. J., & Moens, M. (2002). Description of the Australian cereal cyst nematode Heterodera australis sp. n. (Tylenchida: Heteroderidae). Russian Journal of Nematology, 10, 139–148.
Subbotin, S., Sturhan, D., Rumpenhorst, H. J., & Moens, M. (2003). Molecular and morphological characterisation of the Heterodera avenae species complex (Tylenchida: Heteroderidae). Nematology, 5, 515–538. https://doi.org/10.1163/156854103322683247
Subbotin, S. A., Toumi, F., Elekçioğlu, I. H., Waeyenberge, L., & Maafi, Z. T. (2018). DNA barcoding, phylogeny and phylogeography of the cyst nematode species of the Avenae group from the genus Heterodera (Tylenchida: Heteroderidae). Nematology, 20, 671–702. https://doi.org/10.1163/15685411-00003170
Subbotin, S. A., Mundo-Ocampo, M., Baldwin, J. G. (2010). Systematics of Cyst Nematodes (Nematoda: Heteroderinae), Part B. Brill.
Tamura, K., Stecher, G., & Kumar, S. (2021). MEGA11: Molecular evolutionary genetics analysis version 11. Molecular Biology and Evolution, 38, 3022–3027. https://doi.org/10.1093/molbev/msab120
Thorne, G. (1961). Principles of Nematology. McGraw-Hill.
Towns, J., Cockerill, T., Dahan, M., Foster, I., Gaither, K., Grimshaw, A., Hazlewood, V., Lathrop, S., Lifka, D., & Peterson, G. D. (2014). XSEDE: Accelerating scientific discovery. Computing in Science & Engineering, 16, 62–74. https://doi.org/10.1109/MCSE.2014.80
Vanstone, V. A., Hollaway, G. J., & Stirling, G. R. (2008). Managing nematode pests in the southern and western regions of the Australian cereal industry: Continuing progress in a challenging environment. Australasian Plant Pathology, 37, 220–234. https://doi.org/10.1071/AP08020
Wallace, H. R. (1965). The ecology and control of the cereal root nematode. Journal of the Australian Institute of Agricultural Science, 31, 178–186.
Webster, J. M. (1969). The host-parasite relationships of plant-parasitic nematodes. Advances in Parasitology, 7, 1–40. https://doi.org/10.1016/S0065-308X(08)60433-9
White, J. H. (1953). Wind-borne dispersal of potato-root eelworm. Nature, 172, 686–687. https://doi.org/10.1038/172686a0
Whitehead, A. G., & Hemming, J. R. (1965). A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology, 55, 25–38. https://doi.org/10.1111/j.1744-7348.1965.tb07864.x
Winslow, R. D. (1960). Some aspects of the ecology of free-living and plant-parasitic nematodes. In J. N. Sasser & W. R. Jenkins (Eds.), Nematology, Fundamentals and Recent Advances (pp. 341–415). Chapel Hill.
Yuan, H.-X., Sun, J.-W., Yang, W.-X., Xing, X.-P., Wang, Z.-Y., Riley, I. T., & Li, H.-L. (2010). New pathotypes of Heterodera avenae (cereal cyst nematode) from winter wheat in Zhengzhou, Henan, China. Australasian Plant Pathology, 39, 107–111. https://doi.org/10.1071/AP09050
Zohary, D., Hopf, M., & Weiss, E. (2012). Domestication of Plants in the Old World: The origin and spread of domesticated plants in Southwest Asia, Europe, and the Mediterranean Basin. Oxford University Press.
Acknowledgements
We thank all the growers and agronomists who over many years kindly provided samples which were used as part of this study
Funding
Open access funding provided by CSIRO Library Services. This project is supported by Grains Research and Development Corporation, through funding from the Australian Government Department of Agriculture, Fisheries & Forestry, as part of its Rural R&D for Profit program and along with Cotton Research and Development Corporation, Hort Innovation Australia, Wine Australia, Sugar Research Australia and Forest and Wood Products Australia.
Author information
Authors and Affiliations
Contributions
D.C.H was responsible for primary data collection and wrote the main manuscript text and prepared figures and tables. J.L., and S.C. arranged for and provided necessary samples and background knowledge and data for SA and WA, respectively. A.J. provided samples and sequences from VIC. M.K. processed samples and specimens. M.H. helped develop initial ideas for the project. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was signed by all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huston, D.C., Khudhir, M., Lewis, J. et al. DNA barcoding of Australian cereal cyst nematode populations with comments on likely origin and taxonomy (Tylenchoidea: Heterodera). Phytoparasitica 52, 14 (2024). https://doi.org/10.1007/s12600-024-01136-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12600-024-01136-8