Abstract
Soybean thrips, Neohyadatothrips variabilis (Beach), a vector of Soybean Vein Necrosis Virus (SVNV), has been recognized as a widespread pest of soybean [Glycine max (L.) Merr.] in USA and Canada. Several experiments investigated the effect on soybean when infested with soybean thrips with and without SVNV. Experiment 1 compared soybean infested at V1 and V5 growth stages (GS) with soybean thrips without SVNV and noninfested plants. For plants infested at GS V1, plant height, number of seeds, and seed weights were reduced by 50, 80, and 75%, respectively. When plants were infested at GSs V1 and V5, the counts of soybean thrips peaked at 1,803 and 4,214 per plant 4 weeks after infestation (WAI), respectively. Experiment 2 used SVNV-infected soybean thrips and compared soybean infested versus control at GS V1 and V5. For plants infested at GS V1, plant height was reduced 77.8%, and plants died before setting pods. For plants infested at GS V5, height, number of seeds, and seed weights were reduced 56, 78, and 92%, respectively. When plants were infested at GSs V1 and V5, the counts of soybean thrips peaked at 282 and 883 per plant 2 WAI, respectively. Experiment 3, five soybean genotypes were infested with SVNV-infected thrips at GS V1. Number and weight of seeds per plant were higher and with lower visual disease ratings (progressive chlorosis, necrosis and rugosity) for the more resistant genotypes Merschman Kennedy and plant introduction (PI) 171451 than for the more susceptible genotypes: soybean breeding line LD12-12734a (Rag1/2), cv Williamsfield Illini 3509N, and PI 417136.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The soybean thrips, Neohyadatothrips variabilis (Beach), along with other species of thrips [tobacco thrips Frankliniella fusca (Hinds), Western flower thrips F. occidentalis (Pergande), and Eastern flower thrips F. tritici (Fitch)] have been found feeding on soybean (Glycine max (L.) Merr.). Thrips puncture leaves and buds of soybean plants and can transmit plant viruses. Outbreaks of thrips could devastate soybean yields if the weather is dry or when stressors such as herbicide injury occur (Reisig, 2020).
In 2008, Soybean Vein Necrosis Virus (SVNV) was discovered on soybean in Arkansas and Tennessee (Tzanetakis et al., 2009). As SVNV was subsequently recognized as a widespread virus of soybean in USA and Canada (Bloomingdale et al., 2017), there was greater concern about thrips and the virus on soybean productivity. There have been multiple species of thrips identify on soybeans (Irwin et al., 1979; Viteri et al., 2010), and the soybean thrip is the only one reported to be the primer thrip vector of SVNV (Keough et al., 2016). Their bicolored bodies (brown and yellowish) distinguish the soybean thrips from other thrips found on soybean (Hoddle et al., 2012; Lagos-Kutz et al., 2020).
Past studies showed that seeds from soybean plants infected with SVNV had lower protein and oil than noninfected plants reducing premium marketability (Anderson et al., 2017; Irizarry, 2016). In other crops, thrips and viruses are known to negatively impact yield. Onion thrips were shown to reduce onion yields by up to 50% and cause even more damage when they transmitted Iris Yellow Spot Virus (Waiganjo et al., 2008). Sevik (2012) found that Tomato Spotted Wilt Virus (TSWV) caused 42% and 96% reduction in yield and marketable value of tomato, respectively.
Since there is limited information on loss of soybean productivity in the presence of soybean thrips and SVNV, we conducted greenhouse and growth chamber experiments to evaluate the productivity of soybean plants infested with soybean thrips with and without SVNV.
Materials and methods
Cultures of soybean thrips
Methods for soybean thrips culture and colony maintenance were previously described (Lagos-Kutz et al., 2020).
Growth of soybean with non-infected soybean thrips (Experiment 1) and SVNV-infected (Experiment 2) in the greenhouse
Cultivar Williamsfield Illini 3590N was used to complete Experiments 1 and 2. Three seeds were planted in 15 cm pots (Hummert International, Earth City, MO) in a soilless mix (Sunshine Mix, LC1, Sun Gro Horticulture, Inc., Bellevue, WA) with approximately 15 g of slow-release fertilizer pellets 17–5–11, NPK (Osmocote Blends, ICL Fertilizers, Dublin, OH) spread over the surface of each pot. Plants were thinned to a single plant per pot when plants were at VC growth stage (GS) (Fehr et al., 1971). The greenhouse room was illuminated with fluorescent and incandescent lamps (460 μmol m−2 s−1) set for a cycle of 16 h light and 8 h dark per day with an average temperature of 25 °C during the light period and 22 °C in the dark period. The plants were maintained under these conditions until they reached GS V1. Then the cycle was set to 10 h light and 14 h dark per day to promote earlier flowering and earlier maturation. Plants were then placed into a BugDorm-2120F (MegaView Science Co. Ltd., Taichung, Taiwan) to avoid contamination. Additionally, the plants were covered with 6 ml thickness husky black flashing plastic sheeting (Poly-America LP, Grand Prairie, TX) during the 14 h dark period mentioned above. The plants were maintained under these conditions until they reached GS R1and the photoperiod of 16 h light and 8 h dark per day was resumed. At this time the plants were fertilized with 15 g of slow-release fertilizer pellets 13–13–13, NPK (Osmocote Blends, ICL Fertilizers, Dublin, OH) spread over the surface of each pot.
Each experiment was set up as a 1-factor randomized complete block design with six replicates and with infestation timing (V1, V5, and noninfested control) as a factor. Each of the 18 experimental units for each test were confined in separate BugDorms for a total of 18 BugDorms. Experiment 1 consisted of infesting soybean plants with thrips without SVNV and plants without thrips. In experiment 2, plants were infested with SVNV-infected thrips and plants without thrips.
The soybean thrips used to infest plants for both experiments were from separate growth chamber stocks maintained and routinely assayed for SVNV. For each experiment, the thrips infestation treatments were done by adding two pots of thrip-infested plants from the growth chamber stocks that contained multiple life stages of either noninfected or SVNV-infected soybean thrips to each corresponding experimental unit, allowing the thrips to freely move from heavily infested plants onto the clean experimental plants.
The counts of soybean thrips were done bi-weekly starting 2 weeks after infestation (WAI) and ended at 8 WAI. Plants were evaluated when thrips were counted using a visual severity rating for disease and insect leaf foliar feeding damage using a pre-transformed rating scale (Little & Hills, 1978) from 0 to 5, where 0 = no visible symptoms, 1 = 1 to 10% of the canopy affected, 2 = 11 to 35% of the canopy affected, 3 = 36 to 65% of the canopy affected, 4 = 66 to 90% of the canopy affected, and 5 = 91 to 100% of the canopy affected. Plant height was recorded, and seeds were harvested (counted and weighed) when plants were at R8 GS. In addition, unifoliate leaf tissue of each plant from experiments 1 and 2 were sampled to detect SVNV by using dot blot immunobinding assay (modified method of Srinivasan & Tolin, 1992).
Growth of soybean genotypes with soybean thrips infected with SVNV (Experiment 3)
Three seeds of each soybean genotype were planted in 12.7 cm pots (Hummert International, Earth City, MO) in a soilless mix (Sunshine Mix, LC1, Sun Gro Horticulture, Inc., Bellevue, WA) with 10 g of slow- release fertilizer pellets 17–5–11, NPK (Osmocote Blends, ICL Fertilizers, Dublin, OH) spread over the surface of each pot. Plants were thinned to a single plant per pot at Ve GS.
Plants treated with thrips were infested with 20 females of SVNV-infected soybean thrips when plants reached V1 GS. Each plant was isolated in a 10- × 30-cm cylindrical plastic cage with a 4-mm wall thickness and two 8- × 18-cm side windows sealed with a silk fabric material with 0.1-mm apertures (Hill et al., 2010) for 1 WAI. After 24 h of infestation, 1 g per pot of systemic insecticide, Imidacloprid 1% granular (OHP, Inc., Bluffton, SC), was added to kill soybean thrips. This experiment was conducted in a PGR15 plant growth chamber (Conviron, Winnipeg, Canada) under 10 h light (high out T8s lamp, F48T8/TL841/HO/ALTO, Philips, Mesquite, TX) and 14 h dark per day to promote early flowering and early maturation.
This experiment was set up in a 2-factor completely randomized design with three replicates, five soybean genotypes (Merschman Kennedy 1436RR2 and Williamsfield Illini 3590N, soybean breeding line LD12-12734a (Rag1/2), PI 171451, and PI 417136) and two thrips infestation (with SNVV-infected thrips or without) as the factors for a total of 30 single-plant experimental units.
Similar data (plant height) were collected at harvest and a posteriori (seed counts and weight) as described for experiments 1 and 2. Progress of SVNV symptoms on each plant was recorded. In addition, leaf tissues were sampled from a leaflet of the bottom and top fully expanded trifoliolate of each plant when they reached R1 growth stage or before they died, like for PI 417136, to detect SVNV by using dot blot immunobinding assay.
Statistical analyses
For all experiments, an analysis of variance was performed using JMP Pro 13 (SAS Institute, Cay, NC), using the Fit Model Platform for plant height, number of seeds per plant, and seed weight per plant. For experiments 1 and 2, Infestation timing was a fixed effect and block was considered a random effect. For experiment 3, soybean genotype and infestation were considered fixed effects for plant height, seed count, and seed weight. Rating data were analyzed with soybean genotype as a fixed effect for only the infested treatments. Experiment 2, plants SVNV-infected at V1 GS did not produce seed and were not included in the analyses for number of seed or seed weight. For analysis with significant treatment effects, mean separation was performed using Least Significant Differences (LSD) Means at α = 0.05.
Results
Experiment 1: Growth of soybean infested with soybean thrips not infected with SVNV
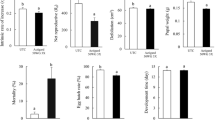
For soybean plants infested at V1 GS, significant differences (P < 0.001) were found compared to the control (noninfested with soybean thrips) for height, number of seeds and seeds weight which were reduced 50%, 80% and 75%, respectively (Fig. 1A–C). For plants infested at V5 GS, significant differences were not found for the number of seeds and seed weights, but the height was reduced 33% compared to the control (Fig. 1A–C). For plants infested at V1 GS, the mean counts of noninfected soybean thrips at 2, 4, 6, and 8 WAI were 782, 1803, 721, and 142, respectively (Fig. 2A). For plants infested at V5 GS, the mean of noninfected soybean thrips at 2, 4, 6 and 8 WAI were 2976, 4214, 2989, and 154, respectively (Fig. 2A). Rating of thrips damage on noninfected soybean thrips at V1 and V5 GSs, and the control were 4, 3 and 0 respectively (Fig. 3A–B). This indicates that soybean thrips without SVNV do not affect the productivity of soybean plants when infested at V5 GS than when infestation occurred at V1 GS.
Growth and productivity of soybean cv. Williamsfield Illini 3590N for control plants (no thrips), of infested soybean thrips either noninfected (A–C) and SVNV-infected (D–F) at V1 and V5 growth stages. Plant height (A and D), seed number (B and E), and seed weight (C and F). Same letters above the bars indicate groupings of means not different (α = 0.05)
Williamsfield Illini 3590N infested with noninfected and Soybean vein necrosis virus (SVNV)-infected soybean thrips. (A) Plant infested at V5 growth stage (GS) with soybean thrips free of SVNV, (B) leaves heavily infested at V5 GS with soybean thrips free of SVNV, (C) control plant without thrips, (D) plant infested at V5 GS with SVNV-infected soybean thrips, and (E) plant infested at V1 GS with SVNV-infected soybean thrips
Experiment 2: Infestation of soybean plants at V1 and V5 stages with SVNV-infected soybean thrips
For plants infested at V1, the height was reduced 77.8% compared to the control (no thrips) (Fig. 1D), and plants died before flowering. For plants infested at V5, significant (P < 0.001) reductions occurred for height (55%), number of seeds (78%), and seeds weight (92%) compared to the control (Fig. 1E–F). For plants infested at V1 stage, the mean of SVNV-infected soybean thrips at 2, 4, 6 and 8 weeks after infestation were 282, 207, 14 and 0, respectively (Fig. 2B). For plants infested at V5, the mean of SVNV-infected soybean thrips at 2, 4, 6 and 8 weeks after infestation were 883, 814, 195, and 51, respectively (Fig. 2B). Visual rating of plants at V1 and V5 GSs, and the control were 5, 4, and 0, respectively (Figs. 3C–E). This indicates that soybean thrips infestation with SVNV at V1 GS can be deadly to soybean plants, and they can cause significant reduction of seeds production and weight when soybean plants were infested at V5 GS.
Leaf dot blots from experiments 1 and 2 with noninfected and SVNV-infected soybean thrips at V1 and V5 GSs were negative and positive as expected according to the treatments (Supplemental Figure S1).
Experiment 3: Growth of soybean genotypes infested with soybean thrips infected with SVNV
Infestation significantly (P < 0.01) reduced plant height by 30.6%, seed count by 33.7%, and seed weight by 43.7% (Fig. 4). Two of the three infested replicates of PI 417136 died due to SVNV prior to harvest. There was a highly significant soybean by infestation interaction (P < 0.01) for response rating. Soybean genotype had a highly significant (P < 0.01) effect on response rating. PI 417136 (4.5), LD12-12734a (3.3), and Williamsfield Illini 3590N (3.3) had significantly higher average ratings than Merschman Kennedy (2.3) and PI 171451 (1.3) (Fig. 5).
Growth and productivity of five soybean genotypes (Merschman Kennedy 1436RR2, Williamsfield Illini 3590N, LD12-12734a, and plant introductions 171451 and 417136) infested with soybean thrips infected with Soybean Vein Necrosis Virus (SVNV) at V1 growth stage. The noninfested plants are free of thrips and SVNV. Same letters above the bars indicate groupings of means not different (α = 0.05)
The visual symptoms including progressive chlorosis and necrosis as well as rugosity which were most pronounced on Williamsfield Illini, LD12-12734a, and PI 417136, as the top trifoliolates showed strong rugosity, less growth including a reduction in plant height (Fig. 6A–J). The dot blots immunobinding assay taken when plants started flowering or about 8 weeks after thrips infestation showed that 100% of leaflets from upper fully expanded trifoliolates were positive for SVNV infection for Williamsfield Illini 3590N and PI 417136, 67% for PI 171451, 33% for Merschman Kennedy 1436RR2, and 0% for LD12-12734a (Supplemental Figure S2). We noted that the first or lowest trifoliolate of soybean genotypes infected with SVNV showed different symptomatology. For example, on cv. Merschman Kennedy 1436RR2 and PI 171451 the resulting lesion was restricted (Fig. 7A, F). However, on cv. Williamsfield Illini 3509N, LD12-12734a, and PI 417136 displayed extensive necrosis (Fig. 7B, D, G), and because of the systemic nature of infection resulted in top trifoliolate displaying strong rugosity (Fig. 7C, E, H). Disease rating of virus symptoms showed differences with PI 171451 rated as 1, Merschman Kennedy rated as 2, LD12-12734a and Williamsfield Illini 3509N rated as 3, and PI 417136 rated as 4. Based on this study, we conclude that the soybean cultivar Merschman Kennedy and PI 171451 are more resistant to SVNV than Williamsfield Illini, PI 417136 and line LD12-12734a.
SVNV-infected and noninfected soybean genotypes maintained in a growth chamber. Plants were photographed when they were at R5-R6 growth stage at about 12 weeks after thrips infestation. The soybean genotypes that look healthy for each treatment were SVNV-infected Merschman Kennedy 1436RR2 (A), LD12-12734a (C), PI 171451 (E), and the noninfected plants (B, D and F respectively). The ones that showed significant differences between each treatment because either it stunted such as SVNV-infected Williamsfield Illini 3590N (G) or died like SVNV-infected PI 417136 (I), compared to the non-infected plants (H and J respectively)
Soybean vein necrosis virus (SVNV) symptoms on five soybean genotypes at mature stage (about 12 weeks after SVNV-infected soybean thrips infestation). (A) Merschman Kennedy 1436RR2: chlorosis and less necrotic surface, (B) Williamsfield Illini 3590N: necrotic leaflet, (C) Williamsfield Illini 3590N: top trifoliolates showing rugosity, (D) LD12-12734a trifoliate: necrosis surface, (E) LD12-12734a: trifoliolates showing rugosity, (F) PI 171451 trifoliate: local necrotic area, (G) PI 417136 trifoliate: expanded necrosis and trifoliolates almost decaying, and (H) PI 417136 trifoliolates: strong rugosity
Discussion
Soybean thrips transmission of SVNV was discovered in Arkansas and Tennessee (Tzanetakis et al., 2009) and not much information has been published about the growth response of soybean infested with soybean thrips with and without SVNV. We used greenhouse and growth chambers to inoculate plants with noninfected and SVNV-infected soybean thrips to evaluate their effect on soybean growth and seed production. We found more of a negative response on plants when they were exposed to thrips (infected or noninfected with SVNV) at V1 GS compared to V5 GS with seed weights per plant being reduced up to 100% compared to non-thrip infested plants. It is obvious from our work that soybean thrips with SVNV can devastate soybean plants under controlled inoculated conditions. In our controlled experiment with five soybean genotypes, there were differences in severity ratings. Among the five soybean genotypes, PI 171451 and Merschamn Kennedy 1436RR2 had reduced severity ratings compared to the other three genotypes. Similar results were found on tomato when affected by specific virus. For example, Tomato Mosaic Virus (TMV) significantly reduced the yield, plant height, fresh and dry shoot, and root weight in susceptible genotypes (Ullah et al., 2017). Sevik (2012) found that Tomato Spotted Wilt Virus (TSWV) caused 42.1% and 95.5% reduction in yield and marketable value of tomato, respectively. Furthermore, TSWV infection in tomato caused significant reductions in weight (26.61%), total number (20.18%), width (10.94%) and length of the fruits (11.93%) in infected plants.
We found that 2-week-old plants or at V1 GS had poorer growth and lower seed productivity when infested with SVNV-infected soybean thrips compared to plants infested when they were 5–6 weeks old. In contrast, experiments on the effect of Cucumber Green Mottle Mosaic Virus on yield indicated that losses of approximately 15% occurred with early infection while later infection had little effect on yield (Fletcher et al., 1969). Similarly, in tomato plants that developed symptoms at 24, 38, or 45 days after transplanting yielded significantly less and produced fewer and smaller tomatoes than those that developed symptoms at 60, 67, and 74 days after transplanting (Moriones et al., 1998). In cowpea plant height and production of leaves were reduced when plants were infected at 10 days after planting than the ones inoculated at 30 days with three unrelated viruses and those plants inoculated 10 days after planting died and did not produce seeds (Kareem & Taiwo, 2007). And in onion crops that early infection of onion yellow dwarf virus significantly reduced the number and weight of seeds/inflorescence compared to late season infection (Maglli et al., 2020). Thus, these studies and ours showed that virus infection have more negative effect on productivity on younger plants than on those of more advanced growth stages.
We also found that higher number of soybean thrips without SVNV do not cause significant effect on productivity than even lower number of soybean thrips with SVNV per soybean plant. It might be that the symptoms of SVNV on soybean, which is necrosis (El-Wahab & El-Shazly, 2017; Zhou & Tzanetakis, 2013), could be the main reason of why fewer SVNV-infected soybean thrips were counted through time. Moreover, Keough et al. (2016) found that SVNV-infected soybean thrips produced 27.50% more offspring compared with soybean thrips without SVNV, but fecundity decreased with virus level increased in them.
In terms of SVNV symptoms, we found through immunization dot blots that SVNV spread systemically on some soybean genotypes (LD12-12734a, cv Williamsfield Illini 3509N, and PI 417136) and locally on cv Merschman Kennedy and PI 171451. The symptoms vary with chlorosis and progressive necrosis days after on leaflet where the thrips did the feeding damage. These symptoms as have been previously observed by other researchers (Zhou & Tzanetakis, 2013; El Wahab et al., 2017). But rugosity symptoms were newly observed especially on those with systemic spreading, and these plants do not normally grow. More research work needs to be done to understand the resistant mechanism both in terms of genetic and host pathogen molecular interactions.
Data Availability
All data relevant to this study is provided within the manuscript.
References
Anderson, N. R., Irizarry, M. D., Bloomingdale, C. A., Smith, D. L., Bradley, C. A., Delaney, D. P., Kleczewski, N. M., Sikora, E. J., Mueller, D. S., & Wise, K. A. (2017). Effect of soybean vein necrosis on yield and seed quality of soybean. Canadian Journal of Plant Pathology, 39, 334–341.
Bloomingdale C., Irizarry, M. D., Groves, R. L., Mueller, D. S., & Smith, D. L. (2017). Seasonal population dynamics of thrips (Thysanoptera) in Wisconsin and Iowa soybean fields. Journal of Economic Entomology, 110, 133–141.
El-Wahab, A. S. A., & El-Shazly, M. (2017). Identification and characterization of soybean vein necrosis virus (SVNV): A newly isolated thrips-borne tospovirus in Egypt. Journal of Virological Sciences, 1, 76–90.
Fehr, W. R., Caviness, C. E., Burmood, D. T., & Pennington, J. S. (1971). Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sciences, 11, 929–931.
Fletcher, J. T., George, A. J., & Green, D. E. (1969). Cucumber green mottle mosaic virus, its effect on yield and its control in the Lea Valley, England. The Plant Pathology Journal, 18, 16–22.
Hoddle, M. S., Mound, L. A., & Paris, D. L. (2012). Thrips of California. CBIT Publishing, Queensland. https://keys.lucidcentral.org/keys/v3/thrips_of_california.html. Accessed 23 Mar 2023.
Irizarry, M. (2016). Soybean vein necrosis virus: impacts of infection on yield loss and seed quality and expansion of plant host range (Master dissertation). Iowa State University, Ames, IA.
Irwin, M. E., Yeargan, K. V., & Marstow, N. L. (1979). Spatial and seasonal patterns of phytophagous thrips in soybean fields with comments on sampling techniques. Environmental Entomology, 8, 131–140.
Kareem, K. T., & Taiwo, M. O. (2007). Interactions of viruses in cowpea: Effects of growth and yield parameters. Virology Journal, 4, 15.
Keough, S., Han, J., Shuman, T., Wise, K., & Nachappa, P. (2016). Effects of soybean vein necrosis virus on life history and host preference of its vector, Neohydatothrips variabilis, and evaluation of vector status of Frankliniella tritici and Frankliniella fusca. Journal of Economic Entomology, 109, 1979–1987.
Lagos-Kutz, D., Pawlowski, M. L., Haudenshield, J. S., Han, J., Domier, L. L., & Hartman, G. L. (2020). Evaluation of soybean for resistance to Neohyadatothrips variabilis (Thysanoptera: Thripidae) noninfected and infected with soybean vein necrosis virus. Journal of Economic Entomology, 113, 949–955.
Little, T. M., & Hills, F. J. (1978). Agricultural experimental design and analysis. John Wiley & Sons.
Maglli, A., Tomassoli, L., Tierini, A., Agosteo, G. E., Fontana, A., Pappu, H. R., & Albanese, G. (2020). A survey on the infection of Onion yellow dwarf virus and Iris yellow spot tospovirus in seed and bulb productions systems of onion in Calabria, Italy. European Journal of Plant Pathology, 156, 767–778.
Moriones, E., Aramburu, J., Riudavets, J., Arnó, J., & Laviña, A. (1998). Effect of plant age at time of infection by tomato spotted wilt tospovirus on the yield of field-grown tomato. European Journal of Plant Pathology, 104, 295–300.
Reisig, D. (2020). Thrips on soybean. North Carolina State Extension Publications. https://content.ces.ncsu.edu/thrips-in-soybean. Accessed 23 Mar 2023.
Sevik, M. A., & Arli-Sokmen, M. (2012). Estimation of the effect of Tomato spotted wilt virus (TSWV) infection on some yield components of tomato. Phytoparasitica, 40, 87–93.
Srinivasan, I., & Tolin, S. A. (1992). Detection of three viruses of clovers by direct tissue immunoblotting. Phytopathology, 82, 721.
Tzanetakis, I., Wen, R., Newman, M., & Hajimorad, M. (2009). Soybean vein necrosis virus: A new threat to soybean production in Southeastern United States. Phytopathology, 99, S131.
Ullah, N., Ali, A., Ahmad, M., Fahim, M., Din, N., & Ahmad, F. (2017). Evaluation of tomato genotypes against tomato mosai virus (ToMV) and its effect on yield contributing parameters. Pakistan Journal of Botany, 49, 1585–1592.
Viteri, D., Cabrera, I., & Estévez de Jensen, C. (2010). Identification and abundance of thrips species on soybean in Puerto Rico. International Journal of Tropical Insect Science, 30, 57–60.
Waiganjo, M. M., Mueke, J. M., & Gitonga, L. M. (2008). Susceptible onion growth stages for selective and economic protection from onion thrips infestation. In R. K Prange & S. D. Bishop (Eds.), Proceedings, Symposium: sustainability through integrated and organic horticulture. International symposium of the International Society for Horticultural Science (ISHS), 13–19 August 2006, Seoul, Korea. Publication Acta Horticulturae. 767 (pp. 193–200). International Society for Horticultural Science.
Zhou, J., & Tzanetakis, I. (2013). Epidemiology of soybean vein necrosis associated virus. Phytopathology, 103, 966–971.
Acknowledgements
We thank the United Soybean Board and United States Department of Agriculture-Agricultural Research Service for financial support. We are grateful to Todd Bedford, USDA-ARS, who provided with seeds from the USDA Soybean Germplasm Collection in Urbana, Illinois.
Funding
Funding provided by USDA-ARS (CRIS 5012-22000-022-00D) and United Soybean Board (#2311-209-0601).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, design and/or writing of the manuscript. Study conception was primarily by Glen Hartman and Doris Lagos-Kutz. Material preparation, data collection and analysis were performed by Doris Lagos-Kutz, Michelle Pawlowski and Jaeyeong Han. The first draft of the manuscript was written by Doris Lagos-Kutz and edited primarily by Glen Hartman and Steven Clough. All authors commented and provided some edits on previous versions of the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors do not have any competing interests to report.
Disclaimer
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture, nor any of the other institutions affiliated with this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12600_2023_1070_MOESM1_ESM.tiff
Supplement Figure S1 Dot blot immunobinding assay results from leaf tissue samples from noninfected and SVNV-infected soybean thrips at growth stages V1 and V5 from experiments 1 and 2. (TIFF 12370 KB)
12600_2023_1070_MOESM2_ESM.tiff
Supplement Figure S2 Dot blot immunobinding assay results from leaf tissue samples from SVNV-infected soybean genotypes at V1 growth stage from experiment 3. (TIFF 8623 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lagos-Kutz, D.M., Pawlowski, M.L., Han, J. et al. Reduction in productivity of soybean plants infested with Neohyadatothrips variabilis (Thysanoptera: Thripidae) with and without soybean vein necrosis virus. Phytoparasitica 51, 437–445 (2023). https://doi.org/10.1007/s12600-023-01070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-023-01070-1