Abstract
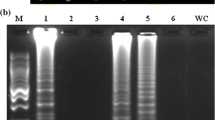
Rice brown spot caused by Bipolaris oryzae (Breda de Haan) is the predominant disease in rice, infecting millions of hectares of cultivated rice, and greatly reduces yields. The various available diagnostic methods for B. oryzae disease detection are time consuming and require sophisticated instruments. Thus, an affordable, rapid and highly specific assay is needed. Here, we report a loop-mediated isothermal amplification (LAMP) assay protocol for specifically detecting B. oryzae by targeting a gene encoding a glycoside hydrolase (GH) family 13 protein. A set of six specific, sensitive LAMP primers were designed, allowing the effective LAMP detection of pathogens after incubation at 62°C for 60 min. Visual detection was performed by the addition of hydroxynapthol blue dye. In a positive reaction, the color changed to intense sky blue, while in a negative reaction, the color remained violet. The developed LAMP assay was highly sensitive, with a detection limit of 100 femtograms. The high specificity of the assay for the detection of B. oryzae was validated. Thus, the LAMP assay protocol provides a rapid, reliable tool for the early detection of B. oryzae under laboratory conditions.






Similar content being viewed by others
References
Alicia, B., Paiva, R. De, Wendland, A., Teixeira, N. C., & Ferreira, M. A. S. V. (2019). Paiva et al_LAMP X.citri, X.phaseoli. 1–37.
Almasi, M. A. (2019). Development of a Color imetr ic Loop-mediated Isothermal Amplification Assay for the Visual Detection of Fusarium oxysporum f . sp . melonis. Horticultural Plant Journal, 5(3), 129–136. https://doi.org/10.1016/j.hpj.2019.01.004
Arutselvan, R., Krishna Reddy, M., & Makeshkumar, T. (2017). Rapid detection of tomato leaf curl Bengaluru virus through loop mediated isothermal amplification assay. VirusDisease, 28(3), 303–308. https://doi.org/10.1007/s13337-017-0385-5
Bartlett, J. M. S., & Stirling, D. (2003). A Short History of the Polymerase Chain Reaction. In PCR Protocols (pp. 3–6). Humana Press. https://doi.org/10.1385/1-59259-384-4:3
Chakrabarti, N. K. (2001). Epidemiology and Disease Management of Brown Spot of Rice in India. In Major Fungal Diseases of Rice (pp. 293–306). Springer. https://doi.org/10.1007/978-94-017-2157-8_21
Choudhary, P., Rai, P., Yadav, J., Verma, S., & Chakdar, H. (2020). OPEN A rapid colorimetric LAMP assay for detection of Rhizoctonia solani AG - 1 IA causing sheath blight of rice. Scientific Reports, 1–19. https://doi.org/10.1038/s41598-020-79117-0
Fukuta, S., Mizukami, Y., Ishida, A., Ueda, J., Hasegawa, M., Hayashi, I., Hashimoto, M., & Kanbe, M. (2004). Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms. European Food Research and Technology, 218(5), 496–500. https://doi.org/10.1007/s00217-003-0862-5
Goto, M., Honda, E., Ogura, A., Nomoto, A., & Hanaki, K. I. (2009). Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques, 46(3), 167–172. https://doi.org/10.2144/000113072
Hansen, Z. R., Knaus, B. J., Tabima, J. F., Press, C. M., Judelson, H. S., Grünwald, N. J., & Smart, C. D. (2016). Loop-mediated isothermal amplification for detection of the tomato and potato late blight pathogen, Phytophthora infestans. Journal of Applied Microbiology, 120(4), 1010–1020. https://doi.org/10.1111/jam.13079
Khan, M., Wang, R., Li, B., Liu, P., Weng, Q., Chen, Q., Montes, H. M., Franco, B., Chen, Q., Weng, Q., & Chen, Q. (2018). Comparative Evaluation of the LAMP Assay and PCR-Based Assays for the Rapid Detection of Alternaria solani. 9(September), 1–11. https://doi.org/10.3389/fmicb.2018.02089
Khush, G. S. (2005). What it will take to Feed 5.0 Billion Rice consumers in 2030. Plant Molecular Biology, 59(1), 1–6. https://doi.org/10.1007/s11103-005-2159-5
Kong, X., Qin, W., Huang, X., Kong, F., Schoen, C. D., Feng, J., Wang, Z., & Zhang, H. (2016). Development and application of loop-mediated isothermal amplification (LAMP) for detection of Plasmopara viticola. Scientific Reports, 6(January), 1–9. https://doi.org/10.1038/srep28935
Laborte, A. G., de Bie, K. C. A. J. M., Smaling, E. M. A., Moya, P. F., Boling, A. A., & Van Ittersum, M. K. (2012). Rice yields and yield gaps in Southeast Asia: Past trends and future outlook. European Journal of Agronomy, 36(1), 9–20. https://doi.org/10.1016/j.eja.2011.08.005
Lan, C., Yao, J., Yang, X., Ruan, H., Jiang, J., Province, F., & Province, F. (2019). Specific and sensitive detection of the guava fruit anthracnose pathogen ( Colletotrichum gloeosporioides ) by loop-mediated isothermal amplification ( LAMP ) assay. 1–31.
Le, D. T., & Vu, N. T. (2017). Progress of loop-mediated isothermal amplification technique in molecular diagnosis of plant diseases. Applied Biological Chemistry, 60(2), 169–180. https://doi.org/10.1007/s13765-017-0267-y
Marimuthu, K., Ayyanar, K., Varagur Ganesan, M., Vaikuntavasan, P., Uthandi, S., Mathiyazhagan, K., & Nagaraj, G. (2020). Loop-mediated isothermal amplification assay for the detection of Plasmopara viticola infecting grapes. Journal of Phytopathology, 168(3), 144–155. https://doi.org/10.1111/jph.12866
Mori, Y., Nagamine, K., Tomita, N., & Notomi, T. (2001). Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochemical and Biophysical Research Communications, 289(1), 150–154. https://doi.org/10.1006/bbrc.2001.5921
Nair, S., Manimekalai, R., Ganga Raj, P., & Hegde, V. (2016). Loop mediated isothermal amplification (LAMP) assay for detection of coconut root wilt disease and arecanut yellow leaf disease phytoplasma. World Journal of Microbiology and Biotechnology, 32(7), 1–7. https://doi.org/10.1007/s11274-016-2078-4
Notomi, T., Okayama, H., Masubuchi, H., Yonekawa, T., Watanabe, K., Amino, N., & Hase, T. (2000a). Loop-mediated isothermal amplification of DNA. In. Nucleic Acids Research, 28, Issue 12.
Panno, S., Matić, S., Tiberini, A., Caruso, A. G., Bella, P., Torta, L., Stassi, R., & Davino, S. (2020). Loop mediated isothermal amplification: Principles and applications in plant virology. Plants, 9(4), 1–28. https://doi.org/10.3390/plants9040461
Prasannakumar, M. K., Parivallal, P. B., Pramesh, D., Mahesh, H. B., & Raj, E. (2021). LAMP-based foldable microdevice platform for the rapid detection of Magnaporthe oryzae and Sarocladium oryzae in rice seed. Scientific Reports, 11(1), 1–10. https://doi.org/10.1038/s41598-020-80644-z
Qi, Y. X., Zhang, X., Pu, J. J., Xie, Y. X., Zhang, H. Q., Huang, S. L., Li, S. L., & Zhang, H. (2009). Nested PCR assay for detection of Corynespora leaf fall disease caused by Corynespora cassiicola. Australasian Plant Pathology, 38(2), 141–148. https://doi.org/10.1071/AP08086
Rocha, D. C., Oliveira, M. B., de Freitas, M. A., & Petrofeza, S. (2017). Rapid detection of Macrophomina phaseolina in common bean seeds using a visual loop-mediated isothermal amplification assay. Australasian Plant Pathology, 46(2), 205–212. https://doi.org/10.1007/s13313-017-0477-0
Savary, S., Teng, P. S., Willocquet, L., & Nutter, F. W. (2006). Quantification and modeling of crop losses: A review of purposes. Annual Review of Phytopathology, 44(March 2015), 89–112. https://doi.org/10.1146/annurev.phyto.44.070505.143342
Sreenivasaprasad, S., Johnson, R., & Chakrabarti, N. K. (2001). Epidemiology and Disease Management of Brown Spot of Rice in India. Major Fungal Diseases of Rice, 293–306. https://doi.org/10.1007/978-94-017-2157-8_21
Tomita, N., Mori, Y., Kanda, H., & Notomi, T. (2008). Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nature Protocols, 3(5), 877–882. https://doi.org/10.1038/nprot.2008.57
Tsunguori Notomi, Hiroto OKayama, Harumi Masubuchi, Toshihiro Yonekawa, Kelko Watanabe, Nobuyuki Amino, T. H. (2000b). Loop mediated isothermal amplification of DNA. Nucleic Acids Research, 28(12).
Wang, Y., Xue, Y., & Li, J. (2005). Towards molecular breeding and improvement of rice in China. Trends in Plant Science, 10(12), 610–614. https://doi.org/10.1016/j.tplants.2005.10.008
Yang, X., Al-attala, M. N., Zhang, Y., Zhang, A., Zang, H., Gu, C., Gao, T., & Chen, Y. (2018). Rapid Detection of Ustilaginoidea virens from Rice using Loop-Mediated Isothermal Amplification Assay. September, 1741–1747. https://doi.org/10.1094/PDIS-01-18-0065-RE
Zhang, S. Y., Dai, D. J., Wang, H. D., & Zhang, C. Q. (2019). One-step loop-mediated isothermal amplification (LAMP) for the rapid and sensitive detection of Fusarium fujikuroi in bakanae disease through NRPS31, an important gene in the gibberellic acid bio-synthesis. Scientific Reports, 9(1), 1–9. https://doi.org/10.1038/s41598-019-39874-z
Zhao, Z., Liu, H., Wang, C., & Xu, J. R. (2014). Correction to Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi [BMC Genomics 14(2013) 274]. BMC Genomics, 15(1). https://doi.org/10.1186/1471-2164-15-6
Acknowledgements
Authors like to acknowledge Science and Engineering Board, EMEQ, New Delhi, India for financial support to carryout this study. We are grateful to Department of Plant Pathology, Tamil Nadu Agricultural University, Coimbatore, India for their full support to complete this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lakshmi, K.R.S., Kamalakannan, A., Gopalakrishnan, C. et al. Loop-mediated isothermal amplification assay: A specific and sensitive tool for the detection of Bipolaris oryzae causing brown spot disease in rice. Phytoparasitica 50, 543–553 (2022). https://doi.org/10.1007/s12600-022-00979-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-022-00979-3