Abstract
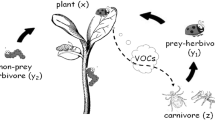
Plants emit volatile secondary metabolites in response to the attacks from herbivorous insects and send signals to carnivorous enemies as reinforcement against the herbivores. Presence of higher carnivores, attracted by the released volatile blends, increases predation pressure on the herbivores. To reduce predation pressure, the herbivore adopts a refuge mechanism. Two natural phenomena are combined by proposing a volatile mediated model-based tritrophic system among the plants, herbivorous insects, and the natural carnivorous enemy, emphasizing the role of plant volatiles and herbivore refuge. In particular, we have highlighted the role of volatile mediated plant’s indirect defense mechanism on its fitness improvement under different ecological consequences, for example, considering different functional responses for herbivores (Holling type II) and carnivorous enemies (Holling type III) associating hiding behavior of herbivores (herbivore refuge) in a tritrophic interaction model. Numerical simulations and analytical treatments are conducted to validate our proposed hypothesis on the tritrophic interaction. Using Isocline method, we show the existence of the interior equilibriums. We illustrate sensitivity analysis of system parameters through Global Sensitivity Analysis using Latin Hypercube Sampling and Partial Ranked Correlation Coefficient. The high-dimensional Bendixson criterion is applied to show global stability of positive equilibrium. We observed two types of alternative states, transcritical bifurcation of limit cycle, and saddle-node bifurcation. High emission of volatiles promotes more stabilized dynamical behaviors, when all three species coexist, thus sustaining the ecological balance in the tritrophic interaction.








Similar content being viewed by others
Data availability
Not applicable.
References
Price, P.W., Bouton, C.E., Gross, P., McPheron, B.A., Thompson, J.N., Weis, A.E.: Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 11(1), 41–65 (1980)
Pichersky, E., Dudareva, N.: Biology of Plant Volatiles. CRC Press, Boca Raton (2020)
Li, T., Holst, T., Michelsen, A., Rinnan, R.: Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nat. Plants 5(6), 568–574 (2019)
Heil, M.: Indirect defence via tritrophic interactions. New Phytol. 178(1), 41–61 (2008)
Dicke, M.: Local and systemic production of volatile herbivore-induced terpenoids: their role in plant-carnivore mutualism. J. Plant Physiol. 143(4–5), 465–472 (1994)
Dudareva, N., Negre, F., Nagegowda, D.A., Orlova, I.: Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25(5), 417–440 (2006)
Kigathi, R.N., Weisser, W.W., Reichelt, M., Gershenzon, J., Unsicker, S.B.: Plant volatile emission depends on the species composition of the neighboring plant community. BMC Plant Biol. 19(1), 58 (2019)
Pan, Y., Wang, Z., Zhao, S.-W., Wang, X., Li, Y.-S., Liu, J.-N., Wang, S., Xi, J.-H.: The herbivore-induced plant volatile tetradecane enhances plant resistance to Holotrichia parallela larvae in maize roots. Pest Manag. Sci. 78(2), 550–560 (2022)
Zhou, S., Jander, G.: Molecular ecology of plant volatiles in interactions with insect herbivores. J. Exp. Bot. 73(2), 449–462 (2022)
Karalija, E., Šamec, D., Dahija, S., Ibragić, S.: Plants strike back: plant volatiles and their role in indirect defence against aphids. Physiol. Plant. 175(1):e13850 (2023)
Schowalter, T.D.: Insect Ecology: An Ecosystem Approach. Academic Press, Cambridge (2022)
Gullan, P.J., Cranston, P.S.: The Insects: An Outline of Entomology. Wiley, Hoboken (2014)
Schmidt, J.O.: Defensive Behavior. Elsevier, Amsterdam (2009)
Das, A., Roy, S.K.: Dynamics of stage-structured prey–predator model with prey refuge and harvesting. Int. J. Model. Simul. 42(6), 966–984 (2022)
Molla, H., Sarwardi, S., Haque, M.: Dynamics of adding variable prey refuge and an Allee effect to a predator–prey model. Alex. Eng. J. 61(6), 4175–4188 (2022)
McNair, J.N.: The effects of refuges on predator–prey interactions: a reconsideration. Theor. Popul. Biol. 29(1), 38–63 (1986)
Ruxton, G.: Short term refuge use and stability of predator–prey models. Theor. Popul. Biol. 47(1), 1–17 (1995)
Collings, J.B.: Bifurcation and stability analysis of a temperature-dependent mite predator–prey interaction model incorporating a prey refuge. Bull. Math. Biol. 57, 63–76 (1995)
Chen, L., Chen, F., Chen, L.: Qualitative analysis of a predator–prey model with Holling type ii functional response incorporating a constant prey refuge. Nonlinear Anal.: Real World Appl. 11(1), 246–252 (2010)
Kar, T.K.: Stability analysis of a prey–predator model incorporating a prey refuge. Commun. Nonlinear Sci. Numer. Simul. 10(6), 681–691 (2005)
Sih, A.: Prey refuges and predator–prey stability. Theor. Popul. Biol. 31(1), 1–12 (1987)
González-Olivares, E., Ramos-Jiliberto, R.: Dynamic consequences of prey refuges in a simple model system: more prey, fewer predators and enhanced stability. Ecol. Model. 166(1–2), 135–146 (2003)
Hassell, M.P.: The Dynamics of Arthopod Predator–Prey Systems. (MPB-13), vol. 13. Princeton University Press, Princeton (2020)
Holt, R.D., Hassell, M.P.: Environmental heterogeneity and the stability of host–parasitoid interactions. J. Anim. Ecol. 89–100 (1993)
Chattopadhayay, J., Sarkar, R., Fritzsche-Hoballah, M.E., Turlings, T.C., Bersier, L.-F.: Parasitoids may determine plant fitness—a mathematical model based on experimental data. J. Theor. Biol. 212(3), 295–302 (2001)
Dicke, M., van Loon, J.J.: Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomologia experimentalis et applicata 97(3), 237–249 (2000)
Liu, Y., Zeng, R., Liu, D., Luo, S., Wu, H., An, M.: Modelling dynamics of plant defence volatiles using the An–Liu–Johnson–Lovett model. Allelopath. J. 18(2), 215 (2006)
Liu, Y.H., Li Liu, D., An, M., Fu, Y.L., Zeng, R.S., Luo, S.M., Wu, H., Pratley, J.: Modelling tritrophic interactions mediated by induced defence volatiles. Ecol. Model. 220(23), 3241–3247 (2009)
Fergola, P., Wang, W.: On the influences of defensive volatiles of plants in tritrophic interactions. J. Biol. Syst. 19(02), 345–363 (2011)
Mondal, R., Kesh, D., Mukherjee, D.: Influence of induced plant volatile and refuge in tritrophic model. Energy Ecol. Environ. 3(3), 171–184 (2018)
Mondal, R., Kesh, D., Mukherjee, D.: Influence of competition in modelling dynamics of plant defense with induced volatile. Model. Earth Syst. Environ. 4(3), 1197–1211 (2018)
Mondal, R., Kesh, D., Mukherjee, D.: Role of induced volatile emission modelling tritrophic interaction. Differ. Equ. Dyn. Syst. (2019). https://doi.org/10.1007/s12591-019-00458-y
Mondal, R., Saha, S., Kesh, D., Mukherjee, D.: Basin transition and alternative states: role of multi-species herbivores-induced volatile in plant–insect interactions. Bull. Math. Biol. 83(10), 100 (2021)
Kalinkat, G., Rall, B.C., Uiterwaal, S.F., Uszko, W.: Empirical evidence of type iii functional responses and why it remains rare. Front. Ecol. Evol. 11, 1033818 (2023)
Erbach, A., Lutscher, F., Seo, G.: Bistability and limit cycles in generalist predator–prey dynamics. Ecol. Complex. 14, 48–55 (2013)
Blower, S.M., Dowlatabadi, H.: Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev./Revue Internationale de Statistique 229–243 (1994)
Huang, Y., Chen, F., Zhong, L.: Stability analysis of a prey–predator model with Holling type III response function incorporating a prey refuge. Appl. Math. Comput. 182(1), 672–683 (2006)
Holling, C.S.: The components of predation as revealed by a study of small-mammal predation of the European Pine Sawfly1. Can. Entomol. 91(5), 293–320 (1959)
Holling, C.S.: The functional response of invertebrate predators to prey density. Mem. Entomol. Soc. Can. 98(S48), 5–86 (1966)
Hassell, M., Lawton, J., Beddington, J.: Sigmoid functional responses by invertebrate predators and parasitoids. J. Anim. Ecol. 249–262 (1977)
Akre, B.G., Johnson, D.M.: Switching and sigmoid functional response curves by damselfly naiads with alternative prey available. J. Anim. Ecol. 703–720 (1979)
Colton, T.F.: Extending functional response models to include a second prey type: an experimental test. Ecology 68(4), 900–912 (1987)
Sarnelle, O., Wilson, A.E.: Type III functional response in daphnia. Ecology 89(6), 1723–1732 (2008)
Kreuzinger-Janik, B., Brüchner-Hüttemann, H., Traunspurger, W.: Effect of prey size and structural complexity on the functional response in a nematode-nematode system. Sci. Rep. 9(1), 5696 (2019)
Kondoh, M.: Foraging adaptation and the relationship between food-web complexity and stability. Science 299(5611), 1388–1391 (2003)
Heckmann, L., Drossel, B., Brose, U., Guill, C.: Interactive effects of body-size structure and adaptive foraging on food-web stability. Ecol. Lett. 15(3), 243–250 (2012)
Murdoch, W.W., Avery, S., Smyth, M.E.: Switching in predatory fish. Ecology 56(5), 1094–1105 (1975)
Hammill, E., Petchey, O.L., Anholt, B.R.: Predator functional response changed by induced defenses in prey. Am. Nat. 176(6), 723–731 (2010)
Zhang, P.J., Wei, J.N., Zhao, C., Zhang, Y.-F., Li, C.Y., Liu, S.S., Dicke, M., Yu, X.P., Turlings, T.C.: Airborne host-plant manipulation by whiteflies via an inducible blend of plant volatiles. Proc. Natl. Acad. Sci. USA 116(15), 7387–7396 (2019)
Sunaryo, M.S.W., Salleh, Z., Mamat, M.: Mathematical model of three species food chain with Holling type-III functional response. Int. J. Pure Appl. Math. 89(5), 647–657 (2013)
Birkhoff, G., Rota, G.: Ordinary Differential Equations. Ginn and Co, Boston (1982)
Perko, L.: Differential Equations and Dynamical Systems, vol. 7. Springer, Berlin (2013)
González-Olivares, E., Ramos-Jiliberto, R.: Dynamic consequences of prey refuges in a simple model system: more prey, fewer predators and enhanced stability. Ecol. Model. 166(1–2), 135–146 (2003)
McNair, J.N.: The effects of refuges on predator–prey interactions: a reconsideration. Theor. Popul. Biol. 29(1), 38–63 (1986)
Freedman, H., Waltman, P.: Persistence in models of three interacting predator–prey populations. Math. Biosci. 68(2), 213–231 (1984)
Li, M.Y., Muldowney, J.S.: A geometric approach to global-stability problems. SIAM J. Math. Anal. 27(4), 1070–1083 (1996)
Sun, C., Loreau, M.: Dynamics of a three-species food chain model with adaptive traits. Chaos Solitons Fractals 41(5), 2812–2819 (2009)
Hastings, A., Powell, T.: Chaos in a three-species food chain. Ecology 72(3), 896–903 (1991)
Ermentrout, B.: Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students, vol. 14. SIAM, Philadelphia (2002)
De Boer, J.G., Hordijk, C.A., Posthumus, M.A., Dicke, M.: Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J. Chem. Ecol. 34(3), 281 (2008)
Jun-Ping, C., Hong-De, Z.: The qualitative analysis of two species predator–prey model with Holling’s type III functional response. Appl. Math. Mech. 7(1), 77–86 (1986)
Mukherjee, D.: Dynamics of defensive volatile of plant modeling tritrophic interactions. Int. J. Nonlinear Sci. 25(2), 76–86 (2018)
Sarwardi, S., Hossain, S., Al Basir, F., Ray, S.: Mathematical analysis of an ecological system using a non-monotonic functional response: effects of gestation delay and predator harvesting. Int. J. Dyn. Control 11(2), 605–618 (2023)
Bairagi, N., Jana, D.: On the stability and Hopf bifurcation of a delay-induced predator–prey system with habitat complexity. Appl. Math. Model. 35(7), 3255–3267 (2011)
Acknowledgements
We thank the anonymous reviewers and the editor for their insightful comments in improving the manuscript. This research of Ritwika Mondal was supported by DST/INSPIRE Fellowship/ 2015/ IF 150747. SS is supported by NPDF, SERB-DST, India, Award ID: PDF/2021/000585.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest or any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, R., Kesh, D., Mukherjee, D. et al. Impact of Volatile Mediated Indirect Defense Response of Plant and Herbivore Refuge in Tritrophic Cascade. Differ Equ Dyn Syst (2024). https://doi.org/10.1007/s12591-024-00682-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12591-024-00682-1