Abstract
We overview the 16-kDa proteolipid mediatophore, the transmembrane c-subunit of the V0 sector of the vacuolar proton ATPase (ATP6V0C) that was shown to mediate the secretion of acetylcholine. Acetylcholine, serotonin, and dopamine (DA) are released from cell soma and/or dendrites if ATP6V0C is expressed in cultured cells. Adeno-associated viral vector-mediated gene transfer of ATP6V0C into the caudate putamen enhanced the depolarization-induced overflow of endogenous DA in Parkinson-model mice. Motor impairment was ameliorated in hemiparkinsonian model mice when ATP6V0C was expressed with DA-synthesizing enzymes. The review discusses application in the future as a potential tool for gene therapy, cell transplantation therapy, and inducible pluripotent stem cell therapy in neurological diseases, from the view point of recent findings regarding vacuolar ATPase.
Similar content being viewed by others
Introduction
Secretion is one of major topics in the physiology [1–6]. Most cells are naturally equipped with the vesicular-soluble N-ethylmaleimide-sensitive factor attachment protein receptor (v-SNARE) that enables vesicular traffic and endo- and exocytosis as well as neurotransmitter release [7, 8]. However, another secretory mechanism has been known since 1966 [9]. In electric eels, acetylcholine (ACh) is released in a paracrine fashion from the soma of electric organ cells and acts on nicotinic ACh receptors in adjacent cells to accumulate a voltage of up to 1000 V [10–12]. Mediatophore, a 16-kDa proteolipid, was originally found in the presynaptic membrane of the Torpedo electric organ. This protein from the electric organ synaptosome was characterized, and its remarkable ACh translocation properties were reported [12–16].
Acetylcholine release by mediatophore
The role of mediatophore in ACh release was revealed by projects in which reconstruction of mediatophore in a variety of cells that had no release machinery for ACh [13]. Israël, Dunat, and their colleagues reported that many cells—splenic cells, hepatocytes, erythrocytes, myeloma cells, and several neuroblastoma cell lines were unable to release ACh in response to calcium (Ca2+) influx [13–16]. Cholinergic neuroblastoma or neuroblastoma-derived hybrid culture cell lines were cloned in Nirenberg’s laboratory at the National Institutes of Health, USA, from 1965 to 1975 [17–20]. Neuroblastoma clones that are inactive with respect to neurotransmitter synthesis were also isolated. N18 neuroblastoma cells are representative of these cells. Other cultured cells, i.e., the C6BU-1 glioma cell line followed by the fibroblastic L cell line, PC12, Neuro 2A, and NG108-15 cells, were competent for ACh release provided that they were loaded with the transmitter [13–16, 21–27]. Interestingly, the ACh release mechanism expressed by the latter cells was Ca2+-dependent, with release elicited by an influx of Ca2+ using the Ca2+ ionophore A23187 [11, 13, 16].
ACh was expected to be produced and released if the choline acetyltransferase (ChAT) gene was overexpressed in inactive N18 neuroblastoma cells. However, ACh release was not detected [22, 23]. Instead, when N18 neuroblastoma cells were transfected with mediatophore and the expressing cells were soaked with ACh, the cells began to secrete ACh [14–16]. Based on these results, it is concluded that ACh release from the neuroblastoma cell soma likely depends on mediatophore. From this view point, the ability to form synapses (ACh release detected in cultured muscle cells) in more than 25 different neuroblastoma clones [19–21] was clearly classified into two groups: the synapse formation-positive and -negative groups (Table 1 and see Table 1 of ref. [20]). The positive phenotype was derived from endogenous mediatophore, and the negative phenotype was caused by the absence of mediatophore [14, 15, 23].
Fisher et al. [28] reported that fibroblasts genetically modified to express ChAT became able to release the transmitter on addition of A23187. Some other cell lines, such as NG108-15 cells, expressed voltage-dependent Ca2+ channels that can be activated after depolarization, and so Ca2+-dependent ACh release was elicited by electrical stimulation [24]. Of particular interest is the work of Higashida and colleagues in co-cultures of myocytes and neuroblastoma (Neuro 2A) or hybrid cell lines (NG108-15 hybrid cell line derived by Sendai virus-induced fusion of C-1300 mouse neuroblastoma clone N18TG-2 resistant to 6-thioguanie with rat glioma clone C6Bu-2, resistant to 5-bromodeoxyuridine; NBr-10A hybrid cell line derived from N18TG-2 neuroblastoma x BRL-30E rat liver cell, resistant to 6-thioguanie) [18–24], in which the release was measured as endplate potential of the ACh receptor origin [24]. The ACh release capacity of NG108-15 or NBr-10A hybrid cells seems to be derived from C6Bu-1 glioma or Buffalo rat liver BRL-30E cells equipped with mediatophore, rather than N18TG-2 neuroblastoma cells [19, 20, 23].
Mediatophore as an ortholog of ATPase
Mediatophore was later shown to be an ortholog of the mammalian c-subunit of the V0 transmembrane sector of the vacuolar proton ATPase (ATP6V0C) [10–16, 29; Fig. 1]. ATP6V0C plays a central role in H+ transport as one part of the multi-subunit complex of ATPase [30–39]. Israël and his group initially proposed that mediatophore forms a proteinaceous pore with the six transmembrane c-subunits of ATPase [10, 11, 34]. However, the amino acid sequence and recent understanding of the c ring of the V0 domain suggest high hydrophobicity of the inner part of the ring, which seems unsuitable for a channel pore [31, 32, 36–38]. Therefore, though it is likely that ATP6V0C is permeable to much smaller ions, such as Na+ and K+ [37–39], Israël’s hypothesis regarding the role of ATP6V0C in neurotransmitter release must be revisited. However, more importantly, to discuss the release source, we need to know whether or not some cell lines possess or lack vesicles, the V0 or V1 component of ATPase sector in the above cultured cell lines or in transfected cells with exogenous ATP6V0C. Such data are not available currently.
Schema of major molecular components of vacuolar ATPase (V-ATPase). (Left) a cartoon of whole v-ATPase. ATP hydrolysis is shown at the peripheral V1 domain composed of A, B, C, and other components. The downhill movement of protons is shown in the V0 sector (a, c, d and other components). (Right) six c subunits in the V0 transmembrane sector of the vacuolar proton ATPase. Redrawn from Forgac and his colleages [37, 38]
In addition, a direct interaction between ATP6V0C and the v-SNARE synaptobrevin and associated modulatory effects on ACh release have been reported [13, 33, 34]. Therefore, the role of mediatophore/ATP6V0C should be carefully reconsidered according to recent advances in knowledge regarding vacuolar ATPase.
Serotonin release from NG108-15 cells
The possibility of increasing efflux of other transmitters is of particular interest. NG108-15 neuroblastoma x glioma hybrid cells or NBr-10A neuroblastoma x Buffalo rat liver cell hybrid cells release ACh upon high potassium (K+) depolarizing stimulation and evoke endplate potentials in muscle cells via electrical stimulation of differentiated NG108-15 or NBr-10A cells due to ACh release [20, 22–24; Table 1].
Falk-Vairant et al. demonstrated the presence of endogenous ATP6V0C in NG108-15 cells via western blotting and concluded that the capacity of NG108-15 cells to release ACh from the cell soma and/or dendrites is due to ATP6V0C [15, 34]. We examined whether NG108-15 cells can release serotonin, which is another neurotransmitter. First, NG108-15 cells were soaked with [3H]serotonin, and the cells were filled with [3H]serotonin by uptake from the extracellular medium. After intensive washing, the cells were stimulated with depolarizing high-K+ medium. [3H]Serotonin was released in the presence of but not in the absence of extracellular Ca2+, indicating that this behavior is Ca2+-dependent [40]. NG108-15 cells possess both small clear vesicles and large dense-core vesicles [40, 41]. Therefore, it is not completely clear if ATP6V0C facilitated the exocytosis of serotonin-containing vesicles or the release of free serotonin in the cytoplasm. And an alternative question remains if release is not through ATP6V0C.
Dopamine release from N18 cells expressing ATP6V0C or NG108-15 cells
Next, we examined the possibility of DA release from N18 cells transfected with expression vectors containing the (5′ to 3′) forward or inverse (3′ to 5′) form of ATP6V0C [39, 42]. After transfection, the cells were preloaded for 1 h with 1 μM [3H]DA in the presence of pargyline, a monoamine oxidase inhibitor, added to prevent degradation of [3H]DA [40]. Stimulation with 80 mM K+ in the perfusion medium resulted in a detectable increase in [3H]DA release in a Ca2+-dependent manner [42]. However, when identical experiments were performed in N18 cells transfected with reverse-ATP6V0C, [3H]DA was not released after high-K+ depolarizing stimulation. It is worth mentioning that ACh and DA share an ATP6V0C-dependent mechanism for DA release from the cell soma or dendrites of ATP6V0C-expressing neuroblastoma cells or NG108-15 cells with endogenous ATP6V0C (Table 1).
Effects of ATP6V0C overexpression on dopamine release in the intact mouse brain
The functional roles of ATP6V0C were elucidated in vivo. Although ATP6V0C is expressed in different brain regions (data not shown), ATP6V0C was overexpressed in the mouse brain using adeno-associated virus (AAV) vectors individually harboring cDNAs of rat ATP6V0C, reverse-ATP6V0C, and ATP6V0C-GFP. Infection with these viruses in the substantia nigra of the intact mouse brain using the microinjection technique [43] revealed ATP6V0C-GFP expression in both tyrosine hydroxylase (TH)-positive neurons and TH-negative cells, which were other non-neuronal cells, probably astrocytes (see Fig. 1 in ref. [42]).
Armed with this information, DA overflow from the synaptic cleft in the mouse striatum after treatment with AAV vectors [42] in the substantia nigra was measured using the in vivo microdialysis method [43]. DA release was significantly higher in mice infected with AAV-ATP6V0C under both resting (5 mM extracellular K+ concentration) and depolarizing (50 mM) conditions (see Fig. 2 in ref. [42]). The result indicates that the facilitated DA release is likely from dopaminergic nerve terminals, not from the soma of striatal neurons. In contrast, viral infection with the reverse direction of ATP6V0C had little or no effect on DA release, with levels comparable to the control (sham-operated with phosphate-buffered saline (PBS) injection) mice.
Behavior in hemiparkinsonian mice with or without ATP6V0C
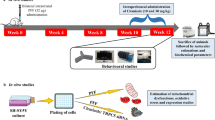
First, we generated hemiparkinsonian mice. A 2-μL aliquot of 6-hydroxydopamine (6-OHDA) dissolved in 0.02 % ascorbic acid in saline was injected into the substantia nigra over a period of 2 min at a rate of 1 μL/min (total injected amount: 28 μg of 6-OHDA) [42]. The needle was left in place for an additional 3 min and then withdrawn slowly. The control animals received PBS in the substantia nigra according to the same procedure.
The motor performance of mice with 6-OHDA-induced lesions in the unilateral substantia nigra was examined based on the latency to fall from an accelerated rotating rod (rotarod test). If the fall latency increased, we judged the change as an effect of recovery from motor impairment. Four AAV vectors individually containing three DA synthetic enzymes (tyrosine hydroxylase (TH), or aromatic l-amino acid decarboxylase (AADC), or GTP cyclohydrolase I (GCH)) and either one of the forward or reverse form of ATP6V0C were infected into the lesion side of the caudoputamen. 6-OHDA-lesioned mice treated with three DA-synthesizing enzymes and ATP6V0C showed significantly improved performance on the rotarod (134.2 ± 14.6 s) in comparison with mice treated with three DA-related genes plus reverse-ATP6V0C (78.1 ± 17.0 s, P < 0.01, n = 5 each, ANOVA (see Fig. 2 in ref. [42]). Recovery was much greater in these mice than in those treated with only these three enzymes alone (75.6 ± 17.2 s) or PBS-treated control mice (24.4 ± 12.2 s) (P < 0.01 and 0.001, respectively, in comparison with the value obtained for mice treated with all three enzymes plus ATP6V0C). The observed recoveries of mice treated with the three enzymes plus ATP6V0C or its reverse form were approximately 73 and 36 % of that observed in the wild-type mice (174.5 ± 12.4 s), respectively [42].
Amphetamine-induced rotations in hemiparkinsonian mice with or without ATP6V0C overexpression
d-Amphetamine-induced rotation is a strong predictor of nigral TH cell loss [44, 45]. Three weeks after lesion generation, the mice exhibited ipsilateral turning induced by the intraperitoneal injection of 3 μg/kg of d-amphetamine [42]. The number of rotations was significantly decreased in mice transfected with the three enzymes, the three enzymes plus reverse-ATP6V0C, and the three enzymes plus ATP6V0C in comparison to control mice injected with PBS (Table 2). In contrast, in sham-operated mice, no rotation was induced by d-amphetamine [42].
Overview of gene or cell transplantation therapy for neurodegenerative diseases
In both rodent and non-human primate models of Parkinson’s disease (PD), viral vector-mediated gene delivery of one enzyme (AADC) or three DA-synthesizing enzymes (TH, AADC, and GCH) into the striatum has been shown to ameliorate motor symptoms, with efficient signal transduction of putaminal neurons [42, 46–54]. Many clinical trials (phase I and phase II) of gene therapy for PD were performed using AAV vectors [55]. In these protocols, gene transfer of AADC into the human putamen is usually combined with oral administration of the precursor l-3,4-dihydroxyphenylalanine (l-DOPA) [48, 49]. Most of the transduced cells are medium spiny neurons (MSNs), the principal projection neurons that account for 90–95 % of all neurons in the striatum. After gene transfer, the MSNs synthesize DA in addition to their inherent inhibitory neurotransmitter, γ-aminobutyric acid (GABA). Gene delivery of DA-synthesizing enzymes would be a useful means of supplying DA continuously in the putamen (Fig. 2).
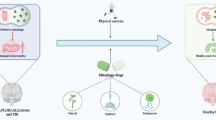
Scheme showing autocrine or paracrine secretion of dopamine from cells expressing dopamine-synthetic enzyme and ATPVC6. Dopamine (DA) is synthetized by AAV-TH/-AADC/-GCH in astrocytes, neurons, and iPSC (yellow) GABAnergic neurons (brown). DA release from the cell soma through the stalk or pore of ATPV0C6. DA released from adjacent cells (paracrine) or GABAnergic neurons (autocrine) binds to DA receptors at the synaptic or non-synaptic domains on GABAnergic neurons. GABAnergic neurons relay signals via DA receptors activated by DA from dopaminergic neurons in the substantia nigra in intact tissue. In the PD brain, DA released in an autocrine or paracrine fashion activates DA receptors
The nerve terminals of dopaminergic neurons of the substantia nigra are selectively degraded and mostly lost in the putamen in the motor phase of chronic PD patients [55]. This observation suggests that even DA synthesized by extrinsic enzymes in the putamen could not be released from the nerve endings of dopaminergic neurons. Rather, cell-somatal release is likely in transduced neurons (Fig. 2), in which the fusion of exocytotic vesicles and the vesicular secretory apparatus are not identical to those observed in the intact nigral nerve terminals [55, 56].
However, the following cautions should be considered. It is possible that injection of the genes encoding ATP6V0C and DA-synthesizing enzymes into the striatum would have augmented the exocytotic DA release from the remaining dopaminergic nerve terminals. Because Jin et al. [42] did not explicitly indicate how many dopaminergic neurons were actually destroyed by their unilateral 6-OHDA injection procedure, although functionally dopaminergic responses were substantially destroyed. In addition, somatic or dendritic release of DA through ATP6V0C in non-dopaminergic neurons or glia is not clearly evident.
It is possible to postulate another mechanism whereby a complex of proteolipid channels involved in SNARE fusion may effectively secrete DA from DA-accumulating cells in the striatum, as described in yeast and Drosophila [7, 57, 58]. ATP6V0C binds to syntaxin in SNARE complexes [59] or interacts directly with the v-SNARE synaptobrevin [33], and ATP6V0C may cooperate with SNARE proteins and vesicles for release during the late stage of fusion.
ATP6V0C as a useful gene in the future
Baseline striatal dopaminergic neurotransmission in the normal striatum is maintained by tonic synaptic and non-synaptic DA release, which are largely independent of changes in neuronal impulse flow in the nigrostriatal pathway. As shown in the previous studies [46, 47, 53], neurons and other types of cells were transfected with AAV, although the majority of transfected cells were neurons. We previously detected efficient baseline and l-DOPA-induced DA release in the AAV-TH/-AADC/-GCH-injected putamen via microdialysis in primates [47], indicating release of the DA synthesized by these extrinsic enzymes. The observation described above, including ATP6V0C, suggests that DA is released from the cell soma of the transfected cells via a non-synaptic mechanism or from non-neuronal elements, such as astro- or microglial cells. DA released from nearby cells binds to the DA receptors on GABAergic neurons and functions in a paracrine or autocrine manner, respectively (Fig. 2).
The concept suggests that ATP6V0C may be useful in future gene or cell transplantation therapy [60, 61]. Gene and cell therapies for other diseases related to ACh and serotonin, such as Alzheimer’s disease, depression, schizophrenia, and syndromic autism, may utilize ATP6V0C in the future [62]. This approach is also applicable for iPSC therapy for similar categories of disease [63–65] because better recovery would be expected if iPSC contains genes for neurotransmitter-synthesizing enzymes and ATP6V0C, because most iPS cells derived from skin fibroblasts are likely silent [62].
Conclusion
We discussed previous results for the efflux of cytosolic and/or vesicular neurotransmitters due to mediatophore/ATP6V0C from the current view involving various possible mechanisms mainly based on recent structural studies [36, 38] and a potential deal of progress in gene therapy and cell transplantation therapy.
References
Katz B et al (1965) Release of acetylcholine from a nerve terminal by electric pulses of variable strength and duration. Nature 207(5001):1097–1098
Südhof TC et al (2011) Synaptic vesicle exocytosis. Cold Spring Harbor Perspective Biol. 3(12):a005637
Neher E et al (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59(6):861–872
Higashida H (2015) Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J Physiol Sci 2015:1–8
Hotta H et al (2014) Non-noxious skin stimulation activates the nucleus basalis of Meynert and promotes NGF secretion in the parietal cortex via nicotinic ACh receptors. J Physiol Sci 64(4):253–260
Daikoku E et al (2015) Zebrafish mutants of the neuromuscular junction: swimming in the gene pool. J Physiol Sci 65(3):217–221
Almers W (2001) Fusion needs more than SNAREs. Nature 409(6820):567–568
Neher E (2015) Merits and limitations of vesicle pool models in view of heterogeneous populations of synaptic vesicles. Neuron 87(6):1131–1142
Sheridan MN et al (1966) The subcellular fractionation of the electric organ of Torpedo. Z Zellforsch Mikrosk Anat 74(3):293–307
Marchbanks RM et al (1971) Aspects of acetylcholine metabolism in the electric organ of Torpedo marmorata. J Neurochem 18(3):439–448
Israël M et al (1986) Purification of a presynaptic membrane protein that mediates a calcium-dependent translocation of acetylcholine. Proc Nat Acad Sci USA 83(23):9226–9230
Israël M et al (1991) Evidence for an association of the 15-kDa proteolipid of mediatophore with a 14-kDa polypeptide. J Neurochem 57(6):2047–2053
Malo M et al (2003) Expression of the acetylcholine release mechanism in various cells and reconstruction of the release mechanism in non-releasing cells. Life Sci 72(18–19):2029–2038
Falk-Vairant J et al (1996) Evoked acetylcholine release expressed in neuroblastoma cells by transfection of mediatophore cDNA. J Neurochem 66(3):1322–1325
Falk-Vairant J et al (1996) Quantal acetylcholine release induced by mediatophore transfection. Proc Nat Acad Sci USA 93(11):5203–5207
Morel N et al (2015) The membrane domain of vacuolar H+ATPase: a crucial player in neurotransmitter exocytotic release. Cell Mol Life Sci 72(13):2561–2573
Amano T et al (1972) Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Nat Acad Sci USA 69(1):258–263
McGee R et al (1978) Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Nat Acad Sci USA 75(3):1314–1318
Nirenberg M et al (1983) Synapse formation by neuroblastoma hybrid cells. Cold Spring Harbor Symp Quant Biology 48(2):707–715
Nirenberg M et al (1983) Modulation of synapse formation by cyclic adenosine monophosphate. Science 222(4625):794–799
Zhong ZG et al (1995) Overexpression of choline acetyltransferase reconstitutes discrete acetylcholine release in some but not all synapse formation-defective neuroblastoma cells. J du Physiologie (Paris) 89(3):137–145
Zhong ZG et al (1995) Discrete acetylcholine release from neuroblastoma or hybrid cells overexpressing choline acetyltransferase into the neuromuscular synaptic cleft. Neurosci Res 22(1):81–88
Higashida H (2012) A personal view from a long-lasting collaborator on the research strategies of Marshall Nirenberg. Neurochem Int 61(6):821–827
Nelson P et al (1976) Synapse formation between clonal neuroblastoma X glioma hybrid cells and striated muscle cells. Proc Nat Acad Sci USA 73(1):123–127
MacDermot J et al (1979) Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Nat Acad Sci USA 76(3):1135–1139
Higashida H et al (1981) Proliferation and synapse formation of neuroblastoma glioma hybrid cells: effects of glia maturation factor. Brain Res 214(2):287–299
Kimura Y et al (1992) Enhanced acetylcholine secretion in neuroblastoma x glioma hybrid NG108-15 cells transfected with rat choline acetyltransferase cDNA. FEBS Lett 314(3):409–412
Fisher LJ et al (1993) In vivo production and release of acetylcholine from primary fibroblasts genetically modified to express choline acetyltransferase. J Neurochem 61(4):1323–1332
Morel M et al (2001) Neurotransmitter release through the V0 sector of V-ATPase. J Neurochem 79(3):485–488
Drory O et al (2006) Structural and functional features of yeast V-ATPase subunit C. Biochim Biophys Acta 1757(5–6):297–303
Inoue T et al (2005) Cysteine-mediated cross-linking indicates that subunit C of the V-ATPase is in close proximity to subunits E and G of the V1 domain and subunit a of the V0 domain. J Biol Chem 280(30):27896–27903
Zhao J et al (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521(7551):241–245
El Far O et al (2011) A role for V-ATPase subunits in synaptic vesicle fusion? J Neurochem 117(4):603–612
Dunant Y et al (2009) Exocytosis, mediatophore, and vesicular Ca2+/H+ antiport in rapid neurotransmission. Ann N Y Acad Sci 1152:100–112
Maxson ME et al (2014) The vacuolar-type H+-ATPase at a glance—more than a proton pump. J Cell Sci 127(Pt 23):4987–4993
Marshansky V et al (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837(6):857–879
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8(11):917–929
Cotter K et al (2015) Recent insights into the structure, regulation, and function of the V-ATPases. Trends Biochem Sci 40(10):611–622
Nezu J et al (1992) Molecular cloning of a rat liver cDNA encoding the 16 kDa subunit of vacuolar H(+)-ATPases: organellar and tissue distribution of 16 kDa proteolipids. J Biochem 112(2):212–219
Furuya S et al (1985) Localization of [3H]serotonin in neuroblastoma x glioma hybrid cells. Brain Res 361(1–2):77–90
Suzuki O et al (1983) Serotonin in neuroblastoma x glioma NG108-15 hybrid cells. Neurochem Int 5(5):599–601
Jin D et al (2012) Dopamine release via the vacuolar ATPase V0 sector c-subunit, confirmed in N18 neuroblastoma cells, results in behavioral recovery in hemiparkinsonian mice. Neurochem Int 61(6):907–912
Jin D et al (2007) CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446(7131):41–45
Iancu R et al (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10
Fleckenstein AE et al (2007) New insights into the mechanism of action of amphetamines. Ann Rev Pharmacol Toxicol 47:681–698
Shen Y et al (2000) Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Hum Gene Ther 11(11):1509–1519
Muramatsu S et al (2002) Behavioral recovery in a primate model of Parkinson’s disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum Gene Ther 13(3):345–354
Muramatsu S et al (2010) Gene therapy for Parkinson’s disease. Strategies for the local production dopamine. Gene Ther Reg 5:57–65
Sun M et al (2004) Coexpression of tyrosine hydroxylase, GTP cyclohydrolase I, aromatic amino acid decarboxylase, and vesicular monoamine transporter 2 from a helper virus-free herpes simplex virus type 1 vector supports high-level, long-term biochemical and behavioral correction of a rat model of Parkinson’s disease. Hum Gene Ther 15(12):1177–1196
Muramatsu S et al (2010) A phase I study of aromatic l-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther 18(9):1731–1735
O’Connor DM et al (2015) Gene therapy for neurodegenerative diseases. Trends Mol Med 21(8):504–512
Chtarto A et al (2013) A next step in adeno-associated virus-mediated gene therapy for neurological diseases: regulation and targeting. Br J Clin Pharmacol 76(2):217–232
Mittermeyer G et al (2012) Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease. Hum Gene Ther 23(4):377–381
Kalia LV et al (2015) Parkinson’s disease. Lancet 386(9996):896–912
Nagatsu T et al (1999) Molecular biology of catecholamine-related enzymes in relation to Parkinson’s disease. Cell Mol Neurobiol 19(1):57–66
Nagatsu T (2007) The catecholamine system in health and disease -Relation to tyrosine 3-monooxygenase and other catecholamine-synthesizing enzymes. Proc Jpn Acad Ser B Phys Biol Sci 82(10):388–415
Hiesinger PR et al (2005) The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121(4):607–620
Peters C et al (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409(6820):581–588
Shiff G et al (1996) Association of syntaxin with SNAP 25 and VAMP (synaptobrevin) in Torpedo synaptosomes. Neurochem Int 29(6):659–667
O’Connor DM et al (2015) Gene therapy for neurodegenerative diseases. Trends Mol Med 21(8):504–512
Olanow CW (2014) Parkinson disease: gene therapy for Parkinson disease—a hope, or a dream? Nat Rev Neurol 10(4):186–187
Fisher LJ et al (1993) Cells engineered to produce acetylcholine: therapeutic potential for Alzheimer’s disease. Ann N Y Acad Sci 695:278–284
Ebrahimi-Fakhari D et al (2015) Autism and the synapse: emerging mechanisms and mechanism-based therapies. Curr Opin Neurol 28(2):91–102
Imaizumi Y et al (2014) Modeling human neurological disorders with induced pluripotent stem cells. J Neurochem 129(3):388–399
Pen AE et al (2016) Current status of treating neurodegenerative disease with induced pluripotent stem cells. Acta Neurol Scand. doi:10.1111/ane.12545
Acknowledgments
We thank M. Ito and N. Takino (Jichi Medical University, Japan) for their help with the production of the AAV vectors. This work was supported by JSPS KAKENHI Grant Number 26293213. This work was also supported by grant-in-aid from “Integrated research on neuropsychiatric disorders” carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and from the Japan Agency for Medical Research and Development (AMED) and also by the industry-Academia Collaborative R&D Programs (COI) from MEXT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. M. owns equity in a gene therapy company (Gene Therapy Research Institution) that commercializes the use of AAV vectors for gene therapy applications. To the extent that the work in this manuscript increases the value of these commercial holdings, S. M. has a conflict of interest.
About this article
Cite this article
Higashida, H., Yokoyama, S., Tsuji, C. et al. Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson’s, Alzheimer’s, and psychiatric diseases. J Physiol Sci 67, 11–17 (2017). https://doi.org/10.1007/s12576-016-0462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-016-0462-3