Abstract
Decreased distensibility of carotid artery is independently associated with the incidence of cardiovascular and cerebrovascular events. Arterial distensibility is determined by vascular tone. Since shear stress is an important driving force of vasodilatory substances production form endothelial cells, we hypothesized that local basal (i.e., resting) arterial blood flow velocity is associated with regional arterial distensibility. To test this hypothesis, we determined the influence of local blood flow velocity on carotid arterial distensibility in cross-sectional study design. In a total of 73 apparent healthy men (18–64 years), carotid arterial properties, including measures of carotid arterial distensibility and BFV at rest, were evaluated via B-mode and Doppler ultrasound imaging and applanation tonometry system. Carotid arterial peak BFV and the absolute and normalized pulsatile BFV significantly correlated with age (r = −0.453 to −0.600, p < 0.0001), whereas mean and minimum BFV were not influenced by age. Distensibility coefficient of carotid artery correlated with peak BFV (r = 0.305, p < 0.01) and more strongly with pulsatile (i.e., systolic minus end-diastolic) BFV (r = 0.406, p < 0.0001) and the normalized pulsatile BFV by time-averaged velocity (r = 0.591, p < 0.0001). Multi-regression analysis revealed that age (β = −0.57, p < 0.0001) was the primary independent determinant for distensibility coefficient. In addition with this, carotid lumen diameter (β = −0.202, p < 0.01) and the normalized pulsatile BFV (β = 0.237, p < 0.05) were significant independent determinants of distensibility coefficient. Qualitatively similar results (although inverse in direction) were obtained by use of β-stiffness index. These results suggest that greater gradient of blood flow velocity during a cardiac cycle are favorably associated with distensibility of carotid artery.
Similar content being viewed by others
Introduction
Large elastic arteries such as the aorta and carotid artery have important roles: expanding as they accept the cardiac ejection from left ventricle with cyclic contraction of the heart, and then recoiling to drive the blood distally. These functions are associated with attenuating excess increases in arterial systolic and pulse pressures, maintaining coronary perfusion, and dampening blood flow fluctuation. Thus, stiffening of large elastic arteries may lead to left ventricular hypertrophy (caused by repeatedly ejecting blood out into stiff arteries) and microvascular damage especially in high-flow vital organs such as the brain and kidneys [1–3], and hence, future incident of cardiovascular and cerebrovascular events [4–7]. Therefore, the elucidation of physiological mechanisms responsible for carotid arterial distensibility regulation might give us a distinct target for the prevention of future cardiovascular and cerebrovascular diseases.
Arterial distensibility is determined by tone of smooth muscle in vessel wall as well as material properties (e.g., elastin and collagen) [8]. There is a growing recognition that shear stress is an important driving force of vasodilatory substances production form endothelial cells [9–11]. Accordingly, we reasoned that local arterial blood flow velocity influences vascular tone via endothelium-derived vasodilatory substances and regulates regional arterial distensibility. The aim of this study was to determine the influence of local blood flow velocity on carotid arterial distensibility in a cross-sectional study design.
Methods
Subjects
A total of 73 apparently healthy men (18–64 years) were recruited. All subjects were free of overt chronic heart diseases assessed by medical history and physical examination and non-medicated. Candidates who smoked in the past 5 years were excluded. All subjects were normotensive (blood pressure <140/90). All experimental procedures and protocols conformed to the Declaration of Helsinki and were approved by the Human Research Institutional Committee of the National Institute of Advanced Industrial Science and Technology before commencement. All subjects gave their written informed consents for participating in this study.
Experimental procedures
All measurements were performed in the morning after an overnight fast except for water intake. Subjects were requested to abstain from caffeinated beverages for 12 h and from strenuous physical activity and alcohol for at least 24 h before the day of the experiment.
Measurements
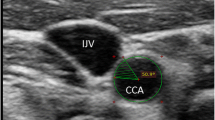
After at least 15 min of resting, hemodynamic variables were measured in supine position. Heart rate (via electrocardiogram) and blood pressure (via oscillometric method) were measured with a vascular testing device (form PWV/ABI; Omron Colin, Kyoto, Japan). Carotid arterial pressure was recorded using an applanation tonometry probe incorporating a high-fidelity strain-gauge transducer (SPT-301, Millar Instruments, Houston, TX, USA) on the left common carotid artery [12]. The pressure signal obtained by tonometry was calibrated by equilibrating the carotid mean arterial and diastolic blood pressures to the brachial mean and diastolic blood pressure measured by an oscillometric device because the baseline levels of carotid blood pressure are subject to hold-down forces [13]. Lumen diameter (from the intima of the far wall the media-adventitia of the near wall) and intima-media thickness (IMT) of carotid artery was evaluated B-mode longitudinal ultrasound images of the right common carotid artery via a duplex ultrasound machine equipped with a high-resolution (10 MHz) linear transducer (SonoSite180PLUS; SonoSite Inc., Bothell, WA, USA). Video clips of carotid arterial pulsation (more than ten beats) were stored as AVI files at 30 flames/s of sampling rate in a personal computer (Precision T5500, Dell Inc., Kawasaki, Japan), and analyzed with image-analysis software (ImageJ 1.48v, NIH, USA). Average diameter in the region of interest (approximately 1 cm × 1 cm square around 1–2 cm proximal to the bifurcation) was computed each flame, and then peak and minimum diameters were extracted in every cardiac cycle. More than five measurements of peak and minimum diameters were averaged and reported, respectively. IMT was measured at end-diastole. To characterize Windkessel function as comprehensively as possible, distensibility coefficient and β-stiffness index (an index of distensibility adjusted for distending pressure) were obtained by the following equations [14, 15]:
where D max and D min are maximal and minimal carotid arterial diameters, respectively. P max and P min are carotid arterial pressure at peak systole and end diastole (i.e., maximal and minimal pressures, respectively). \(\left[ {D_{\hbox{max} } - \, D_{\hbox{min} } } \right]/D_{\hbox{min} }\) was defined as %distension.
Blood flow velocity (BFV) measurements were performed by the Doppler method with the insonation angle <60°. The peak (systolic), minimum (diastolic), pulsatile (peak minus minimum), and mean (time-averaged) BFV were reported as averaged values calculated from successive 7–10 beats of Doppler envelopes. Furthermore, pulsatile BFV was normalized by mean BFV.
Each subject underwent an incremental-graded cycling exercise (with the load increased 15 W every 1 min following a 2-min warm-up period at 20 W) for evaluating aerobic capacity. Throughout the exercise test, expired gas was sampled by using a breath-by-breath method, and oxygen uptake, carbon dioxide output, minute ventilation, respiratory rate, and tidal volume were measured simultaneously with a metabolic cart (AE-300S aero monitor; Minato Ikagaku Co., Tokyo, Japan). Maximal oxygen consumption (VO2max) was estimated by the age-predicted maximum heart rate and the linear regression line between heart rate and oxygen consumption during cycle exercise as we previously described [12].
Statistical analyses
Univariate correlation and multi-regression analyses were performed to determine the relationships among variables of interest. In the multi-regression analysis, to eliminate the influence of multicollinearity, carotid mean (time-averaged) arterial pressure (as a regional steady component) and pulse pressure (as a regional pulsatile component) were only used for candidate determinants of blood pressure. Statistical significance was set a priori at p < 0.05.
Results
Subjects’ physiological characteristics and hemodynamic parameters are presented in Table 1. As shown in Fig. 1, with advancing age, carotid arterial %distension and distensibility coefficient were deceased (r = −0.696 and r = −0.722, p < 0.0001 for both), whereas β-stiffness index was increased (r = 0.661, p < 0.0001). Carotid arterial diastolic diameter and pulse pressure did not correlate with age (r = −0.037 and r = 0.120, respectively). Carotid arterial peak BFV, pulsatile BFV, and the normalized pulsatile BFV significantly correlated with age (r = −0.453 to −0.600, p < 0.0001 for all), whereas mean and minimum BFV were not influenced by age (Fig. 2).
Peak BFV, pulsatile BFV, and the normalized pulsatile BFV was significantly correlated with %distension (r = 0.367–0.624, p < 0.001–0.0001), whereas mean and minimal BFV did not correlate with %distension. No measurements of carotid arterial BFV were correlated with carotid pulse pressure. As shown in Table 2, distensibility coefficient of carotid artery correlated with peak BFV (r = 0.305, p < 0.01), pulsatile BFV (r = 0.406, p < 0.0001), and the normalized pulsatile BFV (r = 0.591, p < 0.0001). Multi-regression analysis revealed that age, heart rate, and lumen diameter, pulse pressure, and the normalized pulsatile BFV of carotid artery were significant independent determinants of distensibility coefficient. Likewise, β-stiffness index correlated with peak BFV (r = −0.281, p < 0.05), pulsatile BFV (r = −0.374, p < 0.01), and the normalized pulsatile BFV (r = −0.530, p < 0.0001). Multi-regression analysis revealed that age, heart rate, estimated VO2max, mean arterial pressure, and carotid arterial lumen diameter and the normalized pulsatile BFV were significant independent determinants of β-stiffness index.
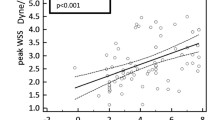
The normalized pulsatile BFV positively and significantly correlated with estimated VO2max (r = 0.479, p < 0.0001, Fig. 3).
Discussion
Aggregate evidence suggests that the lowered distensibility of central elastic arteries could be a risk for cardiovascular and cerebrovascular diseases [4–7]. Accordingly, elucidation of the physiological mechanisms responsible for carotid artery distensibility provides a distinct target for the prevention of future cardiovascular and cerebrovascular diseases. In this study, using a cross-sectional study design, we sought determinants of central arterial distensibility. Major findings are as follows: first, carotid arterial peak and pulsatile BFV decreased with advancing age. Second, indices of carotid arterial distensibility correlated with peak BFV and more strongly with the absolute and normalized pulsatile BFV of carotid artery. Third, multi-regression analysis demonstrated that, in addition to age, the normalized pulsatile BFV was a significant independent determinant of distensibility coefficient. These results suggest that a greater gradient of blood flow velocity during a cardiac cycle at rest is favorably associated with distensibility of the carotid artery.
There is a growing recognition that shear stress is an important driving force of vasodilatory substances production form endothelial cells [9–11]. Based on this notion, we reasoned that local arterial BFV influences vascular tone via endothelium-derived vasodilatory substances and regulates regional arterial distensibility. Expectedly, we found that carotid lumen diameter and normalized pulsatile BFV were significant independent determinants of distensibility coefficient. Qualitatively similar results (although inverse in direction) were obtained by the use of the β-stiffness index. Therefore, carotid arterial distensibility might be favorably influenced by smaller lumen diameter and greater gradient of BFV during a cardiac cycle. These results suggest the contribution of basal (resting) wall shear stress on carotid arterial distensibility. Along with cardiac ejection, basal arterial wall shear stress continuously and chronically exerted by flowing blood on the endothelial surface which to produce vasoactive substances [16]. Thus, our findings have potentially important clinical implications.
Several studies demonstrate outward remodeling of the carotid artery with aging [17], although we observed no significant age-related change in carotid diastolic diameter in the present study. It may lead to decreases of wall shear stress and arterial distensibility. On the other hand, we identified that carotid arterial normalized pulsatile BFV, a significant independent determinant of carotid arterial distensibility, was correlated with aerobic capacity in the present study. These results might be due to high left ventricle ejection and large stroke volume [18]. Therefore, it is necessary to determine whether aerobic exercise training augments carotid arterial blood flow gradient during a cardiac cycle at rest, especially in aged populations from the standpoint of prevention of future cardiovascular and cerebrovascular diseases.
We could speculate possible basal blood flow-mediated substances that may influence arterial distensibility. Nitric oxide (NO), a potent vasodilator, is produced by shear stress from endothelial cells. A recent meta-analysis demonstrates that flow-mediated vasodilation (FMD) of conduit arteries in humans is, at least in part, mediated by NO [11]. Thus, it is plausible that NO contributes to the regulation of basal vascular tone in conduit arteries. However, in young healthy adults, carotid artery distensibility remained unchanged during the systemic NO synthase inhibition in spite of systemic vasoconstriction [19]. Such conflict is possible to be explained by the different hemodynamics (e.g., basal blood flow vs. rapid increase in blood flow). Bellien et al. suggest that cytochrome P450-derived endothelium-derived hyperpolarization factor (EDHF) and/or cyclooxygenase products (such as prostaglandins) are actively secreted in resting conditions by the endothelium of conduit arteries [9]. Furthermore, Gori et al. demonstrate that the radial artery constricts when forearm blood flow is limited by occlusion (i.e., low flow-mediated vasoconstriction [L-FMC]) [16]. FMD is the ability of dilatation in response to the rapid change of blood flow and shear stress, whereas L-FMC is induced by 5 min of occlusions. Thus, diameter change during L-FMC is mediated by the reduced basal blood flow and shear stress. Importantly, this response is blunted by inhibitors of cyclooxygenase and cytochrome P450 (CYP)-derived EDHF but not by the NO synthase inhibitor, suggesting that wall shear stress regulates basal vascular tone of the conduit artery via productions of prostaglandins and EDHF rather than NO. Although NO is considered a key substance of high flow-mediated vasodilation [11], it might not be responsible for basal blood flow and wall shear stress-related basal smooth muscular tone of conduit arteries. Some intervention aimed at modifying flow and/or diameter (e.g., via either CO2 rebreathing, NO donors, COX inhibitors, induction of CYP) is required to elucidate a bit further the mechanisms involved.
Several limitations of this study deserve comments. First, we studied limited subjects cross-sectionally: men older than 65 years and women were not included. Given the well-known limitation of cross-sectional study design, we deemed that cross-sectional study should be confirmed prospectively with the interventional approach as well as with extending subjects of investigation (e.g., women and elderly population). Second, we did not include shear rate into the multiple-regression model as a possible independent variable for carotid arterial distensibility. This is due to avoid multicollinearity: both shear rate and distensibility of carotid arterial are calculated from arterial diameter. Importantly, carotid arterial diameter and pulsatile BFV were significant independent determinants, implying the significant contribution of shear rate to carotid arterial distensibility. On the other hand, hemoglobin concentration and hematocrit, which have potential impacts on blood viscosity and shear stress, were not measured in the present study. As the age-related decrease of hematocrit was reported in men [20], future investigation is needed.
In conclusion, using a cross-sectional study design we sought determinants responsible for carotid arterial distensibility. Our present findings demonstrate that a greater gradient of blood flow velocity during a cardiac cycle is favorably associated with distensibility of the carotid artery.
References
Kelly R, Hayward C, Avolio A, O’Rourke M (1989) Noninvasive determination of age-related changes in the human arterial pulse. Circulation 80:1652–1659
Mitchell GF (2008) Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 105:1652–1660
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ (2010) Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121:505–511
Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC (2006) Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation 113:657–663
van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD (2014) Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol 63:1739–1747
Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327
Yang EY, Chambless L, Sharrett AR, Virani SS, Liu X, Tang Z, Boerwinkle E, Ballantyne CM, Nambi V (2012) Carotid arterial wall characteristics are associated with incident ischemic stroke but not coronary heart disease in the atherosclerosis risk in communities (ARIC) study. Stroke 43:103–108
Nichols W, O’Rourke MF (2005) McDonald’s blood flow in arteries. 5th ed. Theoretical, experimental and clinical principles. Arnold, London
Bellien J, Joannides R, Iacob M, Arnaud P, Thuillez C (2006) Evidence for a basal release of a cytochrome-related endothelium-derived hyperpolarizing factor in the radial artery in humans. Am J Physiol Heart Circ Physiol 290:H1347–H1352
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39:257–265
Green DJ, Dawson EA, Groenewoud HM, Jones H, Thijssen DH (2014) Is flow-mediated dilation nitric oxide mediated?: a meta-analysis. Hypertension 63:376–382
Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T (2005) Effects of aerobic exercise training on the stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55:235–239
Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J (1995) Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 26:48–54
Hirai T, Sasayama S, Kawasaki T, Yagi S (1989) Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80:78–86
Van Bortel LM, Kool MJ, Boudier HAS (1995) Effects of antihypertensive agents on local arterial distensibility and compliance. Hypertension 26:531–534
Gori T, Dragoni S, Lisi M, Di Stolfo G, Sonnati S, Fineschi M, Parker JD (2008) Conduit artery constriction mediated by low flow a novel noninvasive method for the assessment of vascular function. J Am Coll Cardiol 51:1953–1958
Samijo SK, Willigers JM, Barkhuysen R, Kitslaar PJ, Reneman RS, Brands PJ, Hoeks AP (1998) Wall shear stress in the human common carotid artery as function of age and gender. Cardiovasc Res 39:515–522
Tomoto T, Sugawara J, Nogami Y, Aonuma K, Maeda S (2015) The influence of central arterial compliance on cerebrovascular hemodynamics: insights from endurance training intervention. J Appl Physiol 119(5):445–451
Sugawara J, Saito Y, Maeda S, Yoshizawa M, Komine H, Nakamura M, Ajisaka R, Tanaka H (2014) Lack of changes in carotid artery compliance with systemic nitric oxide synthase inhibition. J Hum Hypertens 28:494–499
Coppola L, Caserta F, De Lucia D, Guastafierro S, Grassia A, Coppola A, Marfella R, Varricchio M (2000) Blood viscosity and aging. Arch Gerontol Geriatr 31:35–42
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, (No. 25702045 and 26670116) (JS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
About this article
Cite this article
Tomoto, T., Maeda, S. & Sugawara, J. Influence of blood flow velocity on arterial distensibility of carotid artery in healthy men. J Physiol Sci 67, 191–196 (2017). https://doi.org/10.1007/s12576-016-0455-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12576-016-0455-2