Abstract
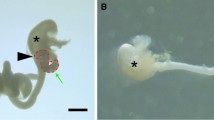
The smooth muscle layer (SML) comprises a significant portion of the intestines and other tubular organs. Whereas epithelial development has recently been extensively studied, SML development has drawn relatively less attention. Previous morphological reports revealed that the inner circular layer (IC) differentiates earlier than the outer longitudinal layer (OL), but detailed development of the SML, including chronological changes in the cell layer number, precise cell orientation, and regional differences in relation to the mesentery, has not been reported. We here observed the development of the SML in the C57BL/6J mouse ileum near the ileocecal junction at embryonic day (E) 13.5, 15.5, and 17.5. By histo-morphometric analyses, in IC, smooth muscle cells (SMCs) were oval-shaped and irregularly arranged in 3–4 layers at E13.5, then adopted an elongated spindle shape and decreased to two cell layers at E15.5 and E17.5. The IC SMC nuclear angle was not vertical, but oriented at 60–80° against the mid-axis of the intestinal lumen. The single SMC layer in OL was observed at E17.5, and the SMC nuclear angle was parallel to the luminal mid-axis. No clear regional difference against the mesentery was observed. Collectively, the findings suggest that development and differentiation of the ileal SML is not simple but regulated in a complex manner and possibly related to the macroscopic organogenesis.





Similar content being viewed by others
References
Brasseur JG, Nicosia MA, Pal A, Miller LS (2007) Function of longitudinal vs circular muscle fibers in esophageal peristalsis, deduced with mathematical modeling. World J Gastroenterol 13:1335–1346
Chamley-Campbell J, Campbell G, Ross R (1979) The smooth muscle cell in culture. Phys Rev 59:1–61
Chevalier NR, de Witte T-M, Cornelissen AJM, Dufour S, Proux-Gillardeax V, Asnacios A (2018) Mechanical tension drives elongational growth of the embryonic gut. Sci Rep 8:5995
Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ (2008) The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Dev Cell 15:134–145
García-Arrarás J, Bello SA, Malavez S (2019) The mesentery as the epicenter for intestinal regeneration. Sem Cell Dev Biol 92:45–54
Geske MJ, Zhang X, Patel KK, Ornitz DM, Stappenbeck TS (2008) Fgf9 signaling regulates small intestinal elongation and mesenchymal development. Development 135:2959–2968
Kosodo Y (2012) Interkinetic nuclear migration: beyond a hallmark of neurogenesis. Cell Mol Life Sci 69:2727–2738
Matsumoto A, Hashimoto K, Yoshioka T, Otani H (2002) Occlusion and subsequent re-canalization in early duodenal development of human embryos: integrated organogenesis and histogenesis through a possible epithelial–mesenchymal interaction. Anat Embryol (Berl) 205:53–65
McHugh KM (1996) Molecular analysis of gastrointestinal smooth muscle development. J Pediatr Gastroenterol Nutr 23:379–394
Miyata T, Okamoto M, Hinoda T, Kawaguchi A (2015) Interkinetic nuclear migration generates and oppose ventricular-zone crowding: insight into tissue mechanics. Front Cell Neuorsci 8:473
Nitta T, Ogawa N, Getachew D, Matsumoto A, Udagawa J, Otani H (2017) Spatiotemporal difference in the mode of interkinetic nuclear migration in the mouse embryonic intestinal epithelium. Shimane J Med Sci 33:79–85
Otani H, Yoneyama T, Hashimoto R, Hatta T, Tanaka O (1993) Ultrastructure of the developing stomach in human embryos. Anat Embryol 187:145–151
Otani H, Udagawa J, Naito K (2016) Statistical analyses in trials for the comprehensive understanding of organogenesis and histogenesis in humans and mice. J Biochem 159:553–561
Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ (2011) On the growth and form of the gut. Nature 476:57–62
Sbarbati R (1982) Morphogenesis of the intestinal villi of the mouse embryo: chance and spatial necessity. J Anat 135:477–499
Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L (2013) Vilification: how the gut gets its villi. Science 342:212–218
Spear PC, Erickson CA (2012) Interkinetic nuclear migration: a mysterious process in search of a function. Dev Growth Differ 54:306–316
Spronck B, Merken JJ, Reesink KD, Kroon W, Delhaas T (2014) Ureter smooth muscle cell orientation in rat is predominantly longitudinal. PLoS ONE 9:e86207
Walton KD, Freddo AM, Wang S, Gumucio DL (2016a) Generation of intestinal surface: an absorbing tale. Development 143:2261–2272
Walton KD, Whidden M, Kolterud A, Shoffner SK, Czerwinski MJ, Kushwaha J, Parmar N, Chandhrasekhar DC, Freddo AM, Schnell S, Gumucio DL (2016b) Vilification in the mouse: Bmp signals control intestinal villus patterning. Development 143:427–436
Yamada M, Udagawa J, Matsumoto A, Hashimoto R, Hatta T, Nishita M, Minami Y, Otani H (2010) Ror2 is required for midgut elongation during mouse development. Dev Dyn 239:941–953
Yamada M, Udagawa J, Hashimoto R, Matsumoto A, Hatta T, Otani H (2013) Interkinetic nuclear migration during early development of midgut and ureteric epithelia. Anat Sci Int 88:31–37
Young HM (2008) On the outside looking in: longitudinal muscle development in the gut. Neurogastroenterol Motil 20:431–433
Acknowledgements
We thank Ms. Yumiko Takeda and Mr. Fumio Satow for their technical help. This work was supported by a MEXT KAKENHI Grant (number 23112006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jahan, E., Rafiq, A.M., Matsumoto, A. et al. Development of the smooth muscle layer in the ileum of mouse embryos. Anat Sci Int 96, 97–105 (2021). https://doi.org/10.1007/s12565-020-00565-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-020-00565-9