Abstract
Euryhaline fishes acclimate to various osmotic environments by changing the direction of water and ion transport between body fluids and environmental waters. Ionocytes in the gills are one of the most important cells for the active ion transport. This study aimed to identify the molecules responsible for apical Cl− transport in rainbow trout ionocytes. Tissue distribution and time-course changes after seawater transfer were analyzed for mRNA expression of slc26a6, cftr1, and cftr2. slc26a6 was specifically expressed in the freshwater gills and decreased after seawater transfer. Both cftr genes were expressed in the gills and higher in seawater; however, the magnitude of expression increase after seawater transfer was greater in cftr1 than in cftr2. These results suggest that Cftr1 is mainly functioned in hypo-osmoregulation and that Cftr2 may also be involved in ion transport under freshwater conditions, such as acid–base regulation. Slc26a6 was localized at the apical membrane of Nkcc1-negative ionocytes only in freshwater-acclimated trout. Apical Cftr1 localization was also identified in most of ionocytes in seawater-acclimated fish. These results indicate that Slc26a6 in freshwater and Cftr1 in seawater contribute to osmoregulatory Cl− transport across the apical membrane of ionocytes in rainbow trout.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rainbow trout Oncorhynchus mykiss is a euryhaline fish in subarctic and cool temperate waters. The wild population of this species is widely distributed in the North Pacific area from northwestern Mexico to the Kamchatka Peninsula and has been artificially introduced into various regions, including the Southern Hemisphere (Okazaki 1983; Smith and Sterley 1989; Molony 2001). There are two types of life histories in rainbow trout: freshwater-resident and anadromous types. Even the latter type spends more than a year in freshwater environment before the migration to the ocean. Rainbow trout are known to survive up to 24–26 °C in freshwater and grow best between 10 °C and 20 °C (Molony 2001). Meanwhile, the high temperature tolerance of salmonids decreases during seawater transfer (Shaughnessy and McCormick 2018; Giroux and Schlenk 2021).
Rainbow trout, like other salmonids, are a very important fish for aquaculture because of their high feed efficiency and growth rate as well as the increasing demand for tasty food. In aquaculture of rainbow trout, they are reared in freshwater until harvest or are transferred to seawater after the juvenile stage. In Japan, rainbow trout have traditionally been raised mainly in inland freshwaters for more than 100 years. Marine trout aquaculture has become more popular due to the increasing demand for larger-sized fish because the growth of rainbow trout is significantly faster in seawater than in freshwater (Teskeredžić et al. 1989). Marine aquaculture of rainbow trout has been practiced in areas where water temperatures are optimal for the high growth performance of this species throughout the year, such as southern coastal area of Chile. However, in relatively warm regions such as Japan, marine trout aquaculture is possible only during the low seawater temperature period from late fall to spring, which is the most limiting factor for the farmed marine trout production in these regions. To maximize the marine culture period of rainbow trout in such area, it is important to determine their ability to withstand high salinity and temperature stress during seawater acclimation. Johnson and Clarke (1988) reported that temperature and salinity synergistically affect the survival rate during seawater acclimation of the trout, that is, information to evaluate the osmoregulatory functions of rainbow trout is necessary to prolong the marine farming period of this species in warm temperate waters.
Osmoregulation is one of the most important physiological functions for teleost species. Although teleosts inhabit various osmotic environments, they maintain their plasma osmolality levels at about one-third of seawater. A gill epithelial cell population called ionocytes plays an important role in active ion transport between body fluids and environmental waters. Ionocytes are also known as mitochondrion-rich cells or chloride cells. The ion-transporting functions of ionocytes are determined by various ion transporters located at either the apical or the basolateral membranes. Na+/K+-ATPase (Nka) at the basolateral membrane generates ionic and electronic gradients across the cell membrane. These gradients provide the driving force for other ion transporters that contribute to ion uptake in freshwater and ion secretion in seawater (Edwards et al. 2013).
The well-accepted model of ion secretion for seawater-type ionocytes is mediated by Nka and Na+, K+, 2Cl− cotransporter 1 (Nkcc1) at the basolateral membrane, and by the cystic fibrosis transmembrane conductance regulator (Cftr) Cl− channel at the apical membrane (Hwang and Lee 2007; Marshall 2011; Kültz and Gilmour 2020). In contrast, ion transport mechanisms of ionocytes in freshwater vary among species (Takei et al. 2014). In euryhaline Mozambique tilapia Oreochromis mossambicus, four types of ionocytes have been identified (Hiroi et al. 2005, 2008; Inokuchi et al. 2008). In freshwater, type-II ionocytes absorb Na+ and Cl− via apical Na+/Cl− cotransporter 2 (Ncc2) and type-III ionocytes absorb Na+ by apical Na+/H+ exchanger 3 (Nhe3) (Inokuchi et al. 2009). In seawater, type-IV ionocytes secrete Na+ through paracellular pathways between neighboring accessory cells and Cl− via basolateral Nkcc1 and apical Cftr. Type-III in freshwater is thought to transform into type-IV in seawater (Hiroi et al. 2008; Choi et al. 2011). A minor population of ionocytes lacking these transporters other than Nka, called type I, has also been identified and is thought to be responsible for transport of other ions (Hiroi et al. 2005; Dymowska et al. 2012).
In rainbow trout, classification of ionocytes based on the expressed transporters is still in progress. The Nhe3-positive ionocytes expressing basolateral Nka and Nkcc1 and apical Nhe3b have been found in both freshwater- and seawater-acclimated rainbow trout. Colocalization of an Rh glycoprotein (Rhcg1, ammonium transporter) and Nhe3b at the apical membrane was also observed, suggesting ammonia-dependent Na+ uptake by Nhe3-positive ionocytes (Hiroi and McCormick 2012). The Nhe3-negative ionocytes, which also lack Nkcc1, are observed mainly in freshwater (Katoh et al. 2008; Hiroi and McCormick 2012). Ncc2, the apical Cl− pathway in tilapia type-II ionocytes, is thought to be absent in the gill of salmonids (Hiroi and McCormick 2012). The Nhe3-positive ionocytes showed basolateral Nka and Nkcc1 both in freshwater and seawater, suggesting that Nhe3-positive ionocytes are analogous to tilapia types-III and -IV and could be equipped with apical Cftr in seawater (Hiroi and McCormick 2012; Takei et al. 2014). However, localization of Cftr proteins by immunohistochemistry has not been successful in salmonids even with homologous antibodies (Takei et al. 2014). The mRNA of slc26a6 has been reported to be highly expressed in the gills of freshwater-acclimated rainbow trout (Boyle et al. 2015; Leguen et al. 2015) and it is very likely that this transporter is responsible for the uptake of Cl− in freshwater, but detailed localization of this protein in the gills has not been elucidated. In short, the molecules responsible for the Cl− transport across the apical membrane have not been identified in both freshwater- and seawater-acclimated rainbow trout.
Salmonids possess two cftr genes, cftr1 and cftr2 (Chen et al. 2001), and it has been reported that the expression level of both genes increases in the gill of chum salmon Oncorhynchus keta after the transfer to seawater during the juvenile stage (Wong et al. 2019). Expression of cftr1 in the gills increased also in rainbow trout after seawater transfer (Gerber et al. 2018). On the other hand, dietary salt loading reduced cftr2 expression in the gill of rainbow trout in freshwater (Kolosov and Kelly 2016). At this time, it is not clear which of these two molecules is mainly responsible for hypo-osmoregulatory Cl− secretion in the gills of salmonids.
The objective of the present study is to examine molecules responsible for the active transport of Cl− in gill ionocytes of rainbow trout. To achieve this goal, we conducted tissue distribution analyses on the expression of slc26a6, cftr1, and cftr2 in rainbow trout acclimated to freshwater or seawater. Time-course changes in the expression of these genes were also examined during seawater transfer. We localized these proteins in the gill filaments of rainbow trout acclimated to freshwater or seawater by whole-mount immunohistochemistry.
Materials and methods
Experimental animals
Fish were maintained in 3000 L tank with recirculating fresh water at 12.5 °C or 15.0 °C and 14:10 h light–dark cycle. The rainbow trout were fed to satiation once a day on commercial trout pellet (Feed One, Yokohama, Japan). The ages of all fish used in this study were less than a year old. Experiments were conducted according to the principles and procedures approved by the Institutional Animal Care and Use Committee of the University of Tokyo.
Sampling for tissue distribution analysis
Tissue distribution analyses for slc26a6, cftr1, and cftr2 were carried out in the brain, pituitary, gill, heart, stomach, pyloric caeca, anterior intestine, posterior intestine, rectum, liver, kidney, spleen, muscle, and skin of rainbow trout. For this experiment, six fish (approximately 300 g) were used. Three of them were transferred directly to 70% seawater for one day and then acclimated to full strength seawater for three weeks. Another three fish for control group were kept in freshwater. The water temperature was maintained 15.0 °C throughout this experiment. Fish were anesthetized with 2-phenoxyethanol and decapitated before tissue removal.
Sampling for time course analysis after seawater transfer
The expression changes of slc26a6, cftr1, and cftr2 in the gills during seawater acclimation were examined. Rainbow trout (approximately 300 g) reared in fresh water at 12.5 °C were directly transferred to full strength seawater and sampled at 0, 1, 5, and 10 days after transfer, with no mortality except for sampling. Gill tissues were collected from six individuals per group at each timepoint. Fish were anesthetized with 2-phenoxyethanol at the time of sampling. After rapid decapitation, gill filaments were removed for quantitative real-time PCR analysis.
RNA extraction and cDNA synthesis
The collected tissues were washed with 0.01 M phosphate-buffered saline (PBS) and homogenized in 1 mL Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) using a bead homogenizer (Micro Smash, Tomy Seiko, Tokyo, Japan). Tissues were stored at −80 °C until processing. The total RNA extraction was carried out with PureLink RNA Mini Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. For each sample, 1 µg of total RNA was reversed-transcribed to cDNA using the High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific) following the manufacturer’s instructions.
Quantitative real-time PCR
The cDNA samples were subjected to quantitative real-time PCR (qPCR) using LightCycler 480 (Roche Diagnostics, Basel, Switzerland) and LightCycler 480 SYBR Green I Master (Roche Diagnostics) with 45 cycles of amplification according to the manufacturer’s instructions. The second derivative maximum method was used to quantify gene expression levels. Amplification specificity of qPCR was confirmed with melting curve analysis (Online Material, Fig. S1). The expression levels of slc26a6, cftr1, and cftr2 were analyzed and 18S rRNA or ef1a was used as internal standards using primer sets (Table 1). Sequences of cftr1 and cftr2, including these entire open-reading frames, were confirmed by Sanger sequencing. Primer sets of slc26a6, cftr1, cftr2, and ef1a were designed using Primer-BLAST by the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Antibodies
For the detection of Nka-immunoreactive ionocytes, the α5 monoclonal antibody (Kiilerich et al. 2011) (Developmental Studies Hybridoma Bank, University of Iowa, Ames, IA, USA) was used. The α5 antibody is a mouse monoclonal antibody against the avian Nka α-subunit and has been widely used to detect branchial Nka in fish (Wong et al. 2016a, b; Perry et al. 2006). To detect Nkcc1, a mouse monoclonal T4 antibody (Developmental studies Hybridoma Bank, University of Iowa) was employed. The T4 antibody has been shown to react with Nkcc1 at the basolateral membrane of ionocytes in rainbow trout (Katoh et al. 2008). Rabbit polyclonal antisera were raised against keyhole limpet hemocyanin-conjugated synthetic peptides corresponding to the amino acid sequence of rainbow-trout Slc26a6 (C + LYLDTDTYEEAK) and Cftr1 (C + IEESLPRGNQTHH) and were purified by affinity chromatography with synthesized peptides (Protein Purify Co. Ltd., Gunma, Japan).
Sampling and immunohistochemistry
For immunohistochemistry, fish (approximately 200 g) were reared in seawater or freshwater for 3 months. The water temperature was maintained at 12.5 °C. Before sampling, fish were euthanized with 2-phenoxyethanol. The second or third gill arches were dissected from freshwater- or seawater-acclimated rainbow trout. Three individuals were used for each condition. Gill tissues were fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer overnight at 4 °C and then stored in 70% ethanol at 4 °C. After washing in PBS containing 0.2% Triton-X (PBST) for 1 h, isolated gill filaments were incubated with PBST containing 10% normal goat serum, 0.1% bovine serum albumin, 0.02% keyhole limpet hemocyanin, and 0.01% sodium azide (NB-PBST) for 1 h.
To determine the localization of Slc26a6 and Cftr1 in the gills of rainbow trout, whole-mount immunohistochemistry for these proteins was performed and immunosignals of these proteins were compared with localization of Nka-immunoreactive ionocytes. The localization of Slc26a6 was also compared with that of Nkcc1.
For triple staining of Slc26a6, Nkcc1, and Nka, the branchial filaments were incubated in NB-PBST containing anti-Slc26a6 antiserum (1:500 diluted) and T4 antibody (1:1000 diluted) overnight at 37 °C. The samples were then incubated with a mixture of goat anti-rabbit immunoglobulin (Ig)G labeled with Alexa Fluor 488, goat anti-mouse IgG labeled with Alexa Fluor 405 (Thermo Fisher Scientific), both diluted 1:500 with PBST. After brief fixation with 4% PFA and rinsing with PBST, the samples were incubated in NB-PBST containing α5 antibody (1:200) labeled with Alexa Fluor 555 by the Zenon Mouse IgG1 Labeling Kit (Thermo Fisher Scientific) overnight at 37 °C.
To detect Cftr1 and Nka in the gill of rainbow trout, the filaments were incubated in NB-PBST containing anti-Cftr1 antiserum (1:500 diluted) and α5 antibody (1:1000 diluted) overnight at 37 °C. The samples were then incubated overnight at 4 °C with a mixture of goat anti-rabbit IgG labeled with Alexa Fluor 488, goat anti-mouse IgG labeled with Alexa Fluor 405 (Thermo Fisher Scientific), both diluted 1:500 with PBST. To reduce autofluorescence, TrueView kit (Vector Laboratories, Burlingame, CA, USA) was used. Briefly, immuno-stained gill filaments were incubated in this reagent diluted to 1:8 with PBS for 40 min at room temperature and washed in PBS for 30 min. The stained samples were observed under a confocal laser scanning microscope (C1, Nikon, Tokyo, Japan).
Statistical analysis
All statistical analyses were performed with GraphPad Prism software, version 7 (GraphPad Software, Boston, MA, USA). Data from the tissue distribution analysis were subjected to Student’s t-test between freshwater and seawater for each of the same tissues. Data that did not pass normality tests were log-transformed. Gene expression levels in seawater transfer experiment were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s (honestly significant difference) HSD test. Results are expressed as mean ± standard error of the mean (SEM) and are considered significantly different when P < 0.05.
Results
Tissue distribution of slc26a6, cftr1, and cftr2 gene expression
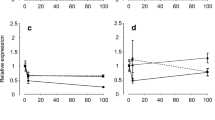
slc26a6 mRNA was predominantly expressed in the gills. Branchial slc26a6 level in freshwater was significantly higher than seawater, with 40 times more mRNA expression (Fig. 1a). cftr1 was highly expressed in the gills, stomach, pyloric caeca, anterior intestine, posterior intestine, and rectum, while little expression was observed in the brain, pituitary, heart, liver, kidney, spleen, muscle, and skin. Significant differences between freshwater and seawater were found in the anterior intestine and the gills. The expression level of cftr1 in the gills was 7.8-fold higher in seawater than in freshwater in anterior intestine, and cftr1 expression level was 1.9-fold higher in seawater (Fig. 1b). Gene expression of cftr2 was observed mainly in the gills, and a significant difference between freshwater and seawater was detected only in the gills. Branchial cftr2 showed a 1.6-fold higher expression level in seawater than in freshwater (Fig. 1c).
The relative expression of slc26a6 (a), cftr1 (b), and cftr2 (c) in the brain, pituitary, gill, heart, stomach, anterior intestine, posterior intestine, rectum, liver, kidney, spleen, muscle, and skin of freshwater- or seawater-acclimated rainbow trout. Data are shown as mean ± SEM (n = 3). * Student’s t-test (P < 0.05), significantly different between freshwater and seawater
Time-course changes in branchial slc26a6, cftr1, and cftr2 mRNA expression after seawater transfer
We tested the branchial transcriptional response of slc26a6, cftr1, and cftr2 during acclimation from freshwater to seawater. Gene expression of slc26a6 in the gills was significantly decreased to 4.3% of the initial level in freshwater until 5 days after seawater transfer. Thereafter, the expression level remained low with no significant change (Fig. 2a). The level of cftr1 mRNA expression at 1 day after seawater transfer was significantly increased to 1.9-fold of the initial value. The expression level on day 5 was 1.5-fold of the initial level, but not significantly different from any timepoint. On day 10, it increased significantly again to 1.8-fold of the freshwater initial level (Fig. 2b). The expression pattern of cftr2 during seawater acclimation was somewhat different from that of cftr1. cftr2 expression significantly increased to1.6-fold at day 1. In contrast to cftr1, the expression of cftr2 was significantly decreased between day 1 and day 5 to the same level of as the initial freshwater expression. cftr2 expression level at day 10 was also not significantly higher than the initial freshwater level (Fig. 2c).
Immunohistochemistry in the gill filaments of rainbow trout
Slc26a6 was detected at the apical membrane of a part of ionocytes in freshwater (Fig. 3a, g), while no clear immunosignal of Slc26a6 were observed in seawater-acclimated fish (Fig. 3b, h). More than half of ionocytes in freshwater were basolaterally stained with T4 antibody (Fig. 3c, e). This immunosignal of Nkcc1 was observed in almost all ionocytes in seawater-acclimated fish (Fig. 3d, f). In freshwater, ionocytes with apical Slc26a6 were different from ones with basolateral Nkcc1. Immunosignals of Cftr1 were detected at the apical membrane of ionocytes in seawater (Fig. 4b). The ionocyte population in seawater-acclimated fish consisted mostly of Cftr1-immunoreactive ionocytes (Fig. 4d), while no immunosignals of Cftr1 were detected in freshwater (Fig. 4a, c).
Discussion
This study examined the molecules involved in Cl− transport in the gills of rainbow trout acclimated to freshwater and seawater. We focused on Slc26a6 for Cl− uptake in the gill ionocytes of freshwater-acclimated rainbow trout. The results of tissue distribution analysis showed that the gene expression of slc26a6 is gill specific and significantly higher in freshwater than in seawater, which suggests the involvement of slc26a6 in the acclimation to a hypo-osmotic environment. This result is consistent with previous studies on the gene in this species (Boyle et al. 2015; Leguen et al. 2015). The cftr genes were noted as candidates for branchial Cl−-secreting molecules in this study. cftr1 was found to be expressed in the gills and gastrointestinal tracts, while the expression of cftr2 was gill specific. Both genes were more highly expressed in seawater than in freshwater, suggesting that these molecules are responsible for Cl− secretion in the gills of the seawater-acclimated trout, as in other teleost species. The gill expression of cftr1 was 7.8 times higher in seawater than in freshwater, while cftr2 was only 1.6 times higher in seawater than in freshwater. These results suggest that cftr2 may have some functionality also in freshwater environments. In Atlantic salmon Salmo salar, it is reported that the highest expression levels of both cftr genes were detected in the intestine as well as in the gills, and that anterior intestinal cftr2 expression was reduced by smoltification or by seawater transfer (Sundh et al. 2014). There may be differences in the tissue distribution of cftr expression, even among closely related salmonids. Cftr protein was localized at the microvilli of the epithelial cells in the intestine of killifish Fundulus heteroclitus and Japanese eel Anguilla japonica (Marshall et al. 2002; Wong et al. 2016a) and is thought to be involved in fluid secretion.
After seawater transfer, branchial slc26a6 expression was gradually decreased, but a significant difference from that in freshwater was not observed until day 5. The stable low expression from day 5 suggests that the expression of slc26a6 was sufficiently downregulated until day 5. Gerber et al. (2018) also showed that branchial slc26a6 expression in smaller rainbow trout (24.5 ± 0.3 g) was significantly decreased after transfer from freshwater to seawater. In their experiment, the significant differences versus freshwater initial were observed as soon as 4 h after seawater transfer. This variation may be due to the size of the fish used. The expressions of both cftr genes were increased at 1 day after seawater transfer. The expression of cftr2 at days 5 and 10 was not significantly different from that in the freshwater initial, while cftr1 at day 10 showed significantly higher expression than that in fish before transfer. In short, changes in expression with salinity stress were clearer in cftr1 than in cftr2. The expression of cftr1 has been reported to increase by hyperosmotic stress in salmonids (Gerber et al. 2018; Handeland et al. 2014). Expression levels of cftr1 and cftr2 in chum salmon fly increased during the smoltification period and were significantly increased by seawater transfer (Wong et al. 2019), which suggests the involvement of cftr1 and cftr2 in seawater acclimation. On the other hand, Mackie et al. (2007) reported no clear upregulation of cftr1 or cftr2 expression in 0-year-old Atlantic salmon transferred to seawater. These findings imply that the responsiveness of cftr expression against a salinity increase in salmonids that have clear smolting period may depend on the degree of smoltification. The results of tissue distribution analysis and seawater transfer in this study suggest that after seawater transfer, the increase in expression is greater in cftr1 than in cftr2 and this trend becomes more apparent over time, and that cftr2 is expressed also in freshwater at a level close to that in seawater. Similarly, the expression of cftr2 in the gills of Atlantic salmon increased by 1.4-fold at 24 h after seawater transfer, but decreased to below freshwater levels after 96 h (Singer et al. 2002). Cftr in mammals is thought to play a role in acid–base regulation via its HCO3−-secreting function (Borowitz 2015; Kim and Steward 2009). The gill of teleost fishes is responsible not only for osmoregulation but also for acid–base regulation, both in freshwater and seawater conditions, and Cftr can be involved in this mechanism by HCO3− secretion in the branchial epithelial cells. Considering that branchial cftr2 in rainbow trout is also expressed in freshwater and that its expression was increased after seawater transfer, a more basic condition than in freshwater, Cftr2 in rainbow trout gill might play a role in acid–base regulation rather than osmoregulation.
Triple staining of Slc26a6, Nkcc1, and Nka proved that there are at least two types of ionocytes in freshwater that can be distinguished by the existence of basolateral Nkcc1. Slc26a6 was localized at the apical membrane of Nkcc1-negative ionocytes and observed only in freshwater-acclimated trout. Colocalization of Nkcc1 and Nhe3 in peanut lectin agglutinin (PNA)-positive ionocytes has been suggested in previous study (Hiroi and McCormick 2012; Ivanis et al. 2008). Therefore, Slc26a6 was thought to exist in PNA-negative ionocytes. Boyle et al. (2015) suggested that branchial Slc26a6 of rainbow trout is a Cl−/HCO3− exchanger based on a pharmacological study. By in situ hybridization, the mRNA of slc26a6 was localized in a part of ionocytes in the gill of freshwater-acclimated rainbow trout (Leguen et al. 2015). Based on these results, Slc26a6 is considered to contribute to acclimation to freshwater environments by absorbing Cl− across the apical membrane in Nkcc1-negative ionocytes. In seawater, no immunoreaction of Slc26a6 was observed and almost all ionocytes possessed basolateral Nkcc1. Nkcc1-negative ionocytes are thought to disappear after seawater transfer with the decrease in slc26a6 expression.
The Cl− secretion model using basolateral Nka, Nkcc, and apical Cftr in ionocytes of seawater-acclimated teleost is widely accepted. However, immunohistochemistry of Cftr in salmonids has not been achieved yet, even with homologous antibodies (Hiroi and McCormick 2012). In the present study, however, immunosignals of Cftr1 protein were detected at the apical membrane of most ionocytes in seawater, while no signal was detected in freshwater. This result strongly suggests that Cftr1 is responsible for the apical Cl− secretion in the gill ionocytes in rainbow trout and that the branchial Cftr-based Cl− secreting system is conserved among salmonids. The antibodies used in this study were raised against amino-acid sequence of Cftr1 because the expression differences between freshwater- and seawater-acclimated trouts was greater in cftr1 than in cftr2 and Cftr1 is thought to be the main Cl− secreting molecule in seawater. Cftr2 may be involved in acid–base regulation by secretion of HCO3−, as described above. Future investigation by in situ hybridization or with specific antibodies on cftr2/Cftr2 are needed to identify its localization in the gills.
Brannen and Gilmour (2018) showed cytosolic carbonic anhydrase is expressed only in PNA-negative ionocytes, which is thought to be the same as Nkcc1-negative and Slc26a6-positive ionocytes. This molecule may affect Slc26a6 activity by regulating the concentration of HCO3−. The mechanism of Cl− transport across the basolateral membrane to body fluids in Slc26a6-positive ionocytes has not been confirmed so far. A chloride channel gene, clc2, has been shown to be expressed in a part of ionocytes of rainbow trout in freshwater (Leguen et al. 2015). Moreover, in zebrafish Danio rerio, Clc2 locates at basolateral membrane of ionocytes and absorb Cl− by cooperating with apical Ncc2 (Wang et al. 2015). These results suggest that Clc2 is most likely a candidate for the Cl− uptake pathway at the basolateral membrane of Slc26a6-positive ionocytes.
In conclusion, the expression of slc26a6 was higher in freshwater while cftr1 and cftr2 were more highly expressed in seawater. Our findings demonstrate localization of Slc26a6 at the apical membrane of Nkcc1-negative ionocytes in freshwater-acclimated rainbow trout. Apical localization of Cftr1 in the gill ionocytes of seawater-acclimated rainbow trout was also revealed. This study provides missing information about protein localization and identifies the molecules responsible for active Cl− transport across the apical membrane in rainbow trout ionocytes. These findings provide fundamental knowledge to understand the stress response against hyperosmotic conditions especially during seawater acclimatization in the marine aquaculture of rainbow trout, and future applications for salmonid farming in seawater are highly expected.
Data availability
All data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Borowitz D (2015) CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 50:S24–S30
Boyle D, Clifford AM, Orr E, Chamot D, Goss GG (2015) Mechanisms of Cl− uptake in rainbow trout: cloning and expression of slc26a6, a prospective Cl−/HCO3− exchanger. Comp Biochem Physiol A Mol Integr Physiol 180:43–50
Brannen M, Gilmour KM (2018) Carbonic anhydrase expression in the branchial ionocytes of rainbow trout. J Exp Biol 221:164582
Chen JM, Cutler C, Jacques C, Bœuf G, Denamur E, Lecointre G, Mercier B, Cramb G, Férec C (2001) A combined analysis of the cystic fibrosis transmembrane conductance regulator: implications for structure and disease models. Mol Biol Evol 18:1771–1788
Choi JH, Lee KM, Inokuchi M, Kaneko T (2011) Morphofunctional modifications in gill mitochondria-rich cells of Mozambique tilapia transferred from freshwater to 70% seawater, detected by dual observations of whole-mount immunocytochemistry and scanning electron microscopy. Comp Biochem Physiol A Mol Integr Physiol 158:132–142
Dymowska AK, Hwang PP, Goss GG (2012) Structure and function of ionocytes in the freshwater fish gill. Respir Physiol Neurobiol 184:282–292
Edwards SL, Marshall WS (2013) Principles and patterns of osmoregulation and euryhalinity in fishes. In: McCormick SD, Farrell AP, Brauner C (eds) Euryhaline fishes. Fish physiology, vol 32. Academic Press, Oxford, pp 1–44
Gerber L, Jensen FB, Madsen SS (2018) Dynamic changes in nitric oxide synthase expression are involved in seawater acclimation of rainbow trout Oncorhynchus mykiss. Am J Physiol Regul Integr Comp Physiol 314:R552–R562
Giroux M, Schlenk D (2021) The effects of temperature and salinity on the endocrinology in two life stages of juvenile rainbow/steelhead trout (Oncorhynchus mykiss). J Fish Biol 99:513–523
Handeland SO, Imsland AK, Nilsen TO, Ebbesson LO, Hosfeld CD, Pedrosa C, Toften H, Stefansson SO (2014) Osmoregulation in Atlantic salmon Salmo salar smolts transferred to seawater at different temperatures. J Fish Biol 85:1163–1176
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184:257–268
Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T (2005) Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+-ATPase, Na+/K+/2Cl-cotransporter and CFTR anion channel. J Exp Biol 208:2023–2036
Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T (2008) Evidence for an apical Na–Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211:2584–2599
Hwang PP, Lee TH (2007) New insights into fish ion regulation and mitochondrion-rich cells. Comp Biochem Physiol A Mol Integr Physiol 148:479–497
Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T (2008) Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Com Biochem Physiol A Mol Integr Physiol 151:151–158
Inokuchi M, Hiroi J, Watanabe S, Hwang PP, Kaneko T (2009) Morphological and functional classification of ion-absorbing mitochondria-rich cells in the gills of Mozambique tilapia. J Exp Biol 212:1003–1010
Ivanis G, Esbaugh AJ, Perry SF (2008) Branchial expression and localization of SLC9A2 and SLC9A3 sodium/hydrogen exchangers and their possible role in acid–base regulation in freshwater rainbow trout (Oncorhynchus mykiss). J Exp Biol 211:2467–2477
Johnsson J, Clarke WC (1988) Development of seawater adaptation in juvenile steelhead trout (Salmo gairdneri) and domesticated rainbow trout (Salmo gairdneri)—effects of size, temperature and photoperiod. Aquaculture 71:247–263
Katoh F, Cozzi RR, Marshall WS, Goss GG (2008) Distinct Na+/K+/2Cl-cotransporter localization in kidneys and gills of two euryhaline species, rainbow trout and killifish. Cell Tissue Res 334:265–281
Kiilerich P, Milla S, Sturm A, Valotaire C, Chevolleau S, Giton F, Terrien X, Fiet J, Fostier A, Debrauwer L, Prunet P (2011) Implication of the mineralocorticoid axis in rainbow trout osmoregulation during salinity acclimation. J Endocrinol 209:221–235
Kim D, Steward MC (2009) The role of CFTR in bicarbonate secretion by pancreatic duct and airway epithelia. J Med Invest 56(Suppl):336–342
Kolosov D, Kelly SP (2016) Dietary salt loading and ion-poor water exposure provide insight into the molecular physiology of the rainbow trout gill epithelium tight junction complex. J Comp Physiol B 186:739–757
Kültz D, Gilmour KM (2020) Iono-and osmoregulation. In: Currie S, Evans DH (eds) The physiology of fishes. CRC Press, pp 63–78
Leguen I, Le Cam A, Montfort J, Peron S, Fautrel A (2015) Transcriptomic analysis of trout gill ionocytes in fresh water and sea water using laser capture microdissection combined with microarray analysis. PLoS ONE 10:e0139938
Mackie PM, Gharbi K, Ballantyne JS, McCormick SD, Wright PA (2007) Na+/K+/2Cl− cotransporter and CFTR gill expression after seawater transfer in smolts (0+) of different Atlantic salmon (Salmo salar) families. Aquaculture 272:625–635
Marshall WS (2011) Mechanosensitive signalling in fish gill and other ion transporting epithelia. Acta Physiol 202:487–499
Marshall WS, Howard JA, Cozzi RRF, Lynch EM (2002) NaCl and fluid secretion by the intestine of the teleost Fundulus heteroclitus: involvement of CFTR. J Exp Biol 205:745–758
Molony B (2001) Environmental requirements and tolerances of rainbow trout (Oncorhynchus mykiss) and Brown trout (Salmo trutta) with special reference to Western Australia: A review. Fish Res Rep West Aust 130:2
Okazaki T (1983) Distribution and seasonal abundance of Salmo gairdneri and Salmo mykiss in the North Pacific Ocean. Jap J Ichthyol 30:235–246
Perry SF, Rivero-Lopez L, McNeill B, Wilson J (2006) Fooling a freshwater fish: how dietary salt transforms the rainbow trout gill into a seawater gill phenotype. J Exp Biol 209:4591–4596
Shaughnessy CA, McCormick SD (2018) Reduced thermal tolerance during salinity acclimation in brook trout (Salvelinus fontinalis) can be rescued by prior treatment with cortisol. J Exp Biol 221:169557
Singer TD, Clements KM, Semple JW, Schulte PM, Bystriansky JS, Finstad B, Fleming IA, McKinley RS (2002) Seawater tolerance and gene expression in two strains of Atlantic salmon smolts. Can J Fish Aquat Sci 59:125–135
Smith GR, Stearley RF (1989) The classification and scientific names of rainbow and cutthroat trouts. Fisheries 14:4–10
Sundh H, Nilsen TO, Lindström J, Hasselberg-Frank L, Stefansson SO, McCormick SD, Sundell K (2014) Development of intestinal ion-transporting mechanisms during smoltification and seawater acclimation in Atlantic salmon Salmo salar. J Fish Biol 85:1227–1252
Takei Y, Hiroi J, Takahashi H, Sakamoto T (2014) Diverse mechanisms for body fluid regulation in teleost fishes. Am J Physiol Regul Integr Comp Physiol 307:R778–R792
Teskeredžić E, Teskeredžić Z, Tomec M, Modrušan Z (1989) A comparison of the growth performance of rainbow trout (Salmo gairdneri) in fresh and brackish water in Yugoslavia. Aquaculture 77:1–10
Wang YF, Yan JJ, Tseng YC, Chen RD, Hwang PP (2015) Molecular physiology of an extra-renal Cl-uptake mechanism for body fluid Cl-homeostasis. Int J Biol Sci 11:1190
Wong MKS, Pipil S, Kato A, Takei Y (2016a) Duplicated CFTR isoforms in eels diverged in regulatory structures and osmoregulatory functions. Comp Biochem Physiol A Mol Integr Physiol 199:130–141
Wong MKS, Pipil S, Ozaki H, Suzuki Y, Iwasaki W, Takei Y (2016b) Flexible selection of diversified Na+/K+-ATPase α-subunit isoforms for osmoregulation in teleosts. Zoological Lett 2:1–22
Wong MKS, Nobata S, Hyodo S (2019) Enhanced osmoregulatory ability marks the smoltification period in developing chum salmon (Oncorhynchus keta). Comp Biochem Physiol A Mol Integr Physiol 238:110565
Acknowledgements
This research was supported by a Grant from the Project of the Bio-oriented Technology Research Advancement Institution (Research program on development of innovative technology) (JPJ007097).
Funding
Open Access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
Conceptualization: Mayu Inokuchi, Soichi Watanabe; Methodology: Mayu Inokuchi, Soichi Watanabe; Formal analysis and investigation: Taisei Kikuchi, Akihiro Hayakawa, Umi Adachi; Writing—original draft preparation: Taisei Kikuchi; Writing—review and editing: Mayu Inokuchi, Soichi Watanabe; Funding acquisition: Atsushi Ido; Resources: Atsushi Ido, Maki Otani, Hiroaki Suetake; Supervision: Soichi Watanabe.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kikuchi, T., Inokuchi, M., Hayakawa, A. et al. Gene expression and protein localization of Cl− transporters, Slc26a6 and Cftr, in the gill ionocytes of rainbow trout. Fish Sci (2024). https://doi.org/10.1007/s12562-024-01809-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12562-024-01809-7