Abstract
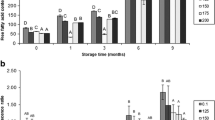
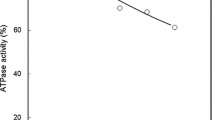
The effect of fish freshness on myosin denaturation during the frozen storage of flounder muscle was studied. The muscle of instantaneously killed flounder was immediately stored at − 20 °C (pre-rigor mortis; day 0), or after storage at 0 °C for 2 days (rigor mortis) or 5 days (post-rigor mortis). Myosin denaturation in frozen stored muscle was studied by measuring Ca2+-ATPase activity, myosin solubility in the presence of salt (salt solubility), and monomeric myosin content. High ATPase activity was maintained in the day-0 samples of instantaneously killed flounder during the early phase of frozen storage. High salt solubility of myosin was also observed in these samples. However, the beneficial effect of frozen storage was lost when the storage period exceeded 2 months. There was no difference in myosin denaturation among the three groups of samples after 2 months of frozen storage. The myosin denaturation profile during the frozen storage of muscle was characterized by high salt solubility in the presence of Mg-ATP, even after ATPase inactivation, especially in the day-0 samples. The salt-soluble fraction contained a large amount of aggregated myosin, as revealed by (NH4)2SO4 fractionation. The monomeric myosin content was explained by Ca2+-ATPase inactivation in the frozen stored meat for all three groups. This study demonstrated that the high quality of flounder meat is maintained during short-term frozen storage, but that the beneficial effects of frozen storage are lost over longer periods of time.








Similar content being viewed by others
References
Amlacher E (1961) Fish As food. Elsevier
FAO (2018) The state of world fisheries and aquaculture 2018. FAO, Rome
Fiske CH, Subbarow Y (1925) Colorimetric determination of phosphate. J Biol Chem 60:375–400
Fukuda Y, Tarakita Z, Arai K (1984) Effect of freshness of chub mackerel on the freeze-denaturation of myofibrillar protein. Nippon Suisan Gakkaishi 50:845–852
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177:751–766
Hu YQ, Zhang JQ, Ebitani K, Konno K (2013) Development of simplified method for extracting ATP-related compounds from fish meat. Nippon Suisan Gakkaishi 79:219–225
Ikeuchi Y, Iwamura K, Suzuki A, Muguruma M, Ito T, Fukazawa T (1990) Heat denaturation of rabbit skeletal G-actin in the presence of ATP. J Sci Food Agric 50:287–296
Iwamoto M, Yamanaka H, Abe H, Watabe S, Hashimoto K (1990) Rigor mortis progress and its temperature-dependency in several marine fishes. Nippon Suisan Gakkaishi 56:93–99
Jantakoson T, Thavaroj W, Konno K (2013) Myosin and actin denaturation in frozen stored kuruma prawn Marsupenaeus japonicus myofibrils. Fish Sci 79:341–347
Jiang LF, Wu SJ (2018) Pullulan suppresses the denaturation of myofibrillar protein of grass carp (Ctenopharyngodon idella ) during frozen storage. Int J Biol Macromol 112:1171–1174
Jiang QQ, Okazaki E, Zheng JW, Que TT, Hu YQ (2018) Structure of northern snakehead (Channa argus) meat: effects of freezing method and frozen storage. Int J Food Prop 21:1166–1179
Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC (1990) Atomic structure of the actin:DNase I complexes. Nature 347:37–44
Kato S, Koseki H, Konno K (1996) Oligomerization of carp myosin which retains its ATPase activity. Fish Sci 62:985–989
Katoh N, Uchiyama H, Tsukamoto S, Arai K (1977) A biochemical study on fish myofibrillar ATPase. Nippon Suisan Gakkaishi 43:857–867
Konno K (1988) G-actin structure revealed by chymotryptic digestion. J Food Biochem 103:386–392
Konno Y, Konno K (2014) Myosin denaturation in “burnt” bluefin tuna meat. Fish Sci 80:381–388
Konno K, Konno Y (2015) Different freeze denaturation of myosin and actin in myofibrils and in muscle. Trans Jpn Soc Refrig Air Condition Eng 32:21–27
Konno K, Ueda YI (1989) Mg-ATPase activity enhancement of carp myofibrils upon thermal treatment. Nippon Suisan Gakkaishi 55:1457–1462
Koseki S, Ootake R, Katoh N, Konno K (2005) Quality evaluation of frozen surimi by using pH stat for ATPase assay. Fish Sci 71:388–396
Kuwahara K, Konno K (2010) Suppression of thermal denaturation of myosin and salt-induced denaturation of actin by sodium citrate in carp (Cyprinus carpio). Food Chem 122:997–1002
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Luan LL, Wang LP, Wu TT, Chen SG, Ding T, Hu YQ (2018) A study of ice crystal development in hairtail samples during different freezing processes by cryosectioning versus cryosubstitution method. Int J Refrig 87:39–46
Matsumoto M, Yamanaka H (1990) Post-mortem biochemical changes in the muscle of kuruma prawn during storage and evaluation of the freshness. Nippon Suisan Gakkaishi 56:1145–1149
Ogata Y, Iwane R, Kimura I (2018) Properties of muscle protein of freeze-thawed olive flounder containing a high concentration of ATP when being frozen. Nippon Suisan Gakkaishi 84:835–842
Paredi ME, Pagano MR, Crupkin M (2010) Biochemical and physicochemical properties of actomyosin and myofibrils from frozen stored flounder (Paralichthys patagonicus) fillets. J Food Biochem 34:983–997
Takahashi M, Yamamoto T, Kato S, Konno K (2005) Species-specific thermal denaturation pattern of fish myosin when heated as myofibrils as studied by myosin subfragment-1 and rod denaturation rates. Fish Sci 71:405–413
The People’s Republic of China Fishery Bureau of the Ministry of Agriculture (2019) China fishery statistics yearbook. China Agriculture Press, Beijing
Wu Y, Zhang J, Shi J, Ebitani K, Konno K (2016) Biphasic IMP decomposition during the storage of flounder muscle at low temperature. Nippon Suisan Gakkaishi 82:342–348
Yoshioka T, Hamai M, Konno K, Arai K (1991) Re-examination of protective effect of ATP on thermal inactivation of myosin Ca-ATPase. Nippon Suisan Gakkaishi 57:143–147
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFD0400603, 2019YFD0902000); Key Technologies for Processing and Quality Improvement of Cold Water Fish, Integration and Demonstration of Key Industrial Technology for Cold Water Fish in Xinjiang Autonomous Region, Major Research & Development Program of Xinjiang Uygur Autonomous Region (2017B01004-4); and the Natural Science Foundation Guidance Plan of Liaoning Province (20180550157).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fan, X., Konno, K., Lin, X. et al. The effect of fish freshness on myosin denaturation in flounder Paralichthys olivaceus muscle during frozen storage. Fish Sci 86, 1111–1120 (2020). https://doi.org/10.1007/s12562-020-01466-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01466-6